PET Imaging in Bronchogenic Carcinoma:
Click here for a discussion of the use of PET FDG imaging in the staging of bronchogenic carcinoma
General:
The PET agent 2-(fluorine-18) fluo-2-deoxy-D-glucose (18-FDG) has been the most studied for evaluation of bronchogenic carcinoma. 18-FDG competes with glucose for facilitated transport into tumor cells and also competes with glucose for phosphorylation by hexokinase. Unlike glucose, however, the phosphorylated form is not further metabolized, it becomes trapped within the cell with little back diffusion or degradation. Uptake of FDG therefore reflects regional rates of exogenous glucose utilization. The agent is useful for imaging bronchogenic carcinoma because lung tumor cells have an increased cellular uptake of glucose due to an increased number of surface glucose transport proteins (increased Glut-1 glucose transporter expression) and a higher rate of glycolysis in comparison to non-neoplastic cells (ie: lung cancer cells have an increased metabolic rate). FDG uptake is related to the degree of cell differentiation and increased FDG uptake is generally associated with greater degrees of de-differentiation [1]. FDG uptake is also related to the proliferation potential of lung cancer as estimated by the proliferating cell nuclear antigen labeling index [1]
FDG PET imaging can have a significant impact on patient management by heightening suspicion for pulmonary malignancy, identifying unsuspected sites of disease, and by guiding selection of a biopsy site. Similarly, a negative PET scan indicates a low likelihood for malignancy and supports the use of conservative management and follow-up [40]. In a study evaluating the impact of FDG PET exam results in the clinical management of patients with suspected or known thoracic malignancies PET scans influenced treatment in 65% of patients and offered new information in 85% [40].
Solitary Pulmonary Nodule Evaluation:
The differential for a solitary pulmonary nodule (SPN) is quite broad, but a large percentage of these lesions may prove to be malignant (especially if the lesion is larger than 2 cm). In the evaluation of an SPN, only 10-20% of patients with malignancy will have sputum positive for cancer [5]. Additionally, up to 30% of patients with negative transthoracic biopsies are ultimately found to have malignancy [5].
Standard uptake value: FDG PET imaging can be used to evaluate indeterminate solitary pulmonary nodules to determine whether the nodule is benign or malignant. A standardized uptake ratio (SUR) or standard uptake value (SUV) is used to determine if a lesion has increased FDG activity. The SUV normalizes the amount of FDG accumulation in a region of interest (ROI) to the total injected dose and the patient's body weight. It provides a means of comparison of FDG uptake between patients [41]. The SUV is calculated by dividing the mean activity within a selected region of interest (in mCi/ml) by the injected dose (in mCi/kg). Modifications of the SUV that may improve the semiquantitative evaluation of FDG uptake include using body surface area or the lean body weight instead of the weight of the patient; this is significant because the accumulation of FDG is higher in muscle than in fat [44]. Additionally, normalization to body surface area or lean body mass potentially reduce the effect of weight loss (which may occur during therapy) on subsequent SUV determinations [76]. It is also important to remember that SUV values may change with time after FDG injection; thus, the time of acquisition after FDG injection must be standardized for the values to be useful [25,76]. Other factors which can affect the SUV include partial-volume effects, patient motion (due to lesion blurring), and the blood glucose level at the time of injection [46,53]. For SUV measurements to be accurate, the PET scanner should be routinely evaluated for quantitative integrity [76].
SUV= Mean selected region activity (mCi/ml)/injected dose (mCi)/body weight (kg)
An SUV greater than 2.5 has been shown to be very sensitive and specific for malignant lesions [13].
Visual analysis of the amount of uptake within a lesion has also been shown to be effective in differentiating benign from malignant lesions [13,47,71]. Uptake greater than blood pool activity (i.e.: the liver or mediastinum typically have an SUV of 2.0) is considered indicative of a malignant lesion, while activity equal to or less than mediastinal blood pool suggests a benign lesion [47]. Overall, visual analysis may be more sensitive for nodules smaller than 1.5 cm in size (although, the improved sensitivity comes at a decreased specificity) [47]. In fact, solid pulmonary lesions with low FDG uptake are probably better evaluated based upon visual inspection [71]. Lesions with visually absent uptake have a very low probability for malignancy, while any visually evident tracer uptake (even faintly visible) within the lesion is associated with about a 60% probability for malignancy [71]. Therefore, any lesion that is visually detectable on FDG PET imaging should be considered carefully for the possibility of malignancy [71,77]. Luckily, most malignant pulmonary nodules with SUV values of less than 2.5 are differentiated adenocarinomas [71].
High sensitivities (82-100%), specificities (67-100%), and accuracy (79-94%) have been reported for the identification of pulmonary malignancy using FDG PET imaging [25,47,50,52,77]. In a prospective multicenter study for the evaluation of pulmonary nodules (larger than 7 mm) felt to be indeterminate for malignancy based upon their conventional imaging appearance, FDG PET had a sensitivity of 92% and a specificity of 90% using SUR data [47]. Visual analysis of the images demonstrated a sensitivity of 98%, but a lower specificity of 69% [47]. However, if the pretest likelihood for malignancy is greater than 50%, the post-test probability for disease will exceed 10% even if the FDG PET scan is negative and histopathologic evaluation will still be required [68]. Combined PET/CT imaging has been shown to have improved sensitivity and specificity for lesion characterization compared to either CT or PET alone [79]. In a comparison to dynamic contrast enhanced CT imaging, PET/CT has been shown to have a higher specificity [77], sensitivity [72], and accuracy [72].
Improved measurement of lesion volume, increased detection of small pulmonary nodules, and improved quantification of FDG uptake can be achieved with respiratory gated PET imaging [54,58,73]. Respiratory motion produces lesion blurring and an apparent increase in lesion size [54]. This results in a decrease in the activity concentration per pixel within the lesion and an underestimation of SUV [54]. The degree of lesion motion is dependent on location within the lung (apical lesions will move little, while lower lung lesions can move considerably) [54]. Respiratory motion also degrades PET/CT fusion imaging and max SUV values can vary up to 30% as a result of CT data acquired during different phases of the respiratory cycle [58,62]. 3D imaging may be required in order to obtain sufficient counts in each gate of the PET study [58]. Optimal implementation of respiratory gating will also require gating of the transmission scan [58].
Dual time imaging: Improved detection of malignant lesions can be obtained by performing dual time point imaging [16,53]. This technique takes advantage of the fact that tracer activity will washout of inflammatory lesions, while malignant lesions retain or increase in FDG activity over time [16,53]. In the study by Matthies et al [53], sensitivity and specificity of a standard SUV measurement greater than 2.5 for malignancy were 80% and 94%, respectively. For dual time imaging, using a threshold of a 10% increase in SUV between the two exams, sensitivity increased to 100% and specificity was 89% [53]. Lesions with a baseline SUV of less than 1.0 have a very high likelihood of being benign and dual time imaging is not required as it may result in false positive exams [53]. A limitation to consider when performing dual time imaging is patient motion which can significantly affect SUV determination, especially for low SUV values. Also- the change in SUV is dependent on reproducibility of the same ROI between both exams [53].
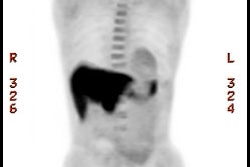
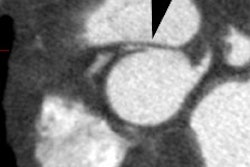
Example: The CT scan in this patient demonstrated a small nodule in the left upper lobe (black arrow). PET-FDG images demonstrate very intense accumulation of the tracer within the lesion (white arrow), which was a non-small cell lung cancer. (Case courtesy of H. Page McAdams MD, Department of Radiology, Duke University Medical Center) |
Lung cancer 1 cm lesion: The images below were from a patient with a 1 cm sized right upper lobe lung cancer. Note the excellent conspicuity of the lesion on FDG PET imaging. The exam was acquired using an ECAT EXACT PET scanner (CTI) with 5 min/bed emission and 2 min/bed segmented transmission. OS-EM iterative reconstruction was used for exam reconstruction. Case courtesy of Mallinckrodt Institute of Radiology/ Barnes Hospital, St. Louis and CTI PET Systems, Inc. |
True negative PET scan: The patient in the scan below had a 1.5 cm left upper lobe nodule (shown below) that could be retrospectively identified on a prior CT scan at which time it measured only 4 mm. A PET scan was performed (below right), but demonstrated no uptake in the lesion (some cardiac activity can be seen more anteriorly). Because the nodule had enlarged from a prior exam, the nodule was resected and found to be a granuloma. Nodules larger than 1.5 cm that are negative on PET scans have a highly likelihood for representing benign or indolent lesions. |
|
False positive results are not uncommon and can be seen with pneumonia [59], infectious granulomas (tuberculosis, histoplasmosis, aspergillosis, cryptococcus, and inflammatory pseudotumor) [36,50,59] or inflammatory processes (sarcoid [59], Wegener's), rheumatoid nodules [13,15], cryptogenic organizing pneumonia (BOOP) [63], nodular pulmonary amyloidosis [59], and an aggressive neurofibroma [8]. Progressive massive fibrosis in silicosis can be markedly positive (likely because of inflammatory cell activity) and cannot be reliably distinguished from lung cancer [63,74]. False positive lung nodule exams are thought to be related to increased glycolytic activity within activated macrophages [3]. Talc pleurodesis also creates a chronic granulomatous reaction which can have increased FDG uptake [59].
False positive PET scan: The patient below had a right middle lobe nodule detected incidentally during abdominal CT scanning. The nodule demonstrated increased FDG uptake on PET imaging. Due to a history of chemical exposure and second hand smoke inhalation the nodule was resected and found to be a granuloma. |
|
Although uncommon, false negative exams can occur. It should be noted that the long term survival of patients with a false-negative PET scan for lung cancer suggests that these tumor behave in an indolent manner [65]. False negative exams tend to occur in four settings [41,73].
1- A neoplasm with low metabolic activity: Bronchoalveolar cell carcinoma (BAC) may have a lower uptake value and can cause a false-negative result in up to 57% of cases [18,21,47,50,60]. Interestingly, Thallium-201 imaging is more commonly positive for BAC [1]. Because bronchoalveolar cell carcinoma is often PET-negative, PET scans should be discouraged if a nodule is predominantly ground-glass [67]. Another pulmonary neoplasm which frequently has a negative FDG PET exam (SUR < 2.5) is a carcinoid tumor (up to 85% of cases) [42,43,50,60]. Mucoepidermoid carcinomas may show little FDG uptake [74]. Although less common, other NSCLC's (particularly well differentiated adenocarcinomas and less commonly squamous cell carcinoma) may also fail to demonstrate significant FDG accumulation [38,47,60]. With the exception of BAC and carcinoid, lung cancers with false-negative PET scans are usually early stage or low malignant potential lesions with an overall favorable prognosis [60]. Indeterminate pulmonary nodules which are negative on FDG PET imaging can be managed conservatively with serial CT evaluation to assess for signs of growth [60].
Bronchoalveolar cell carcinoma (BAC): The images below were from a patient with bronchoalveolar cell carcinoma that presented as a chronic right lung infiltrate. The FDG PET exam was positive in this case despite a higher likelihood of a false negative exams in patients with BAC. Note the most intense area of FDG accumulation corresponds to the area of greatest consolidation on CT imaging. |
|
Atypical carcinoid tumor: The patient presented for evaluation of a right lung nodule. The nodule was positive on PET imaging and was surgically resected. The nodule proved to be an atypical carcinoid tumor. Most carcinoids are not positive on PET imaging, however, atypical carcinoids are more aggressive lesions and this may explain the FDG accumulation. Unfortunately, the patient had ipsilateral hilar lymph node metastatses which were not seen on CT or on PET imaging. |
|
2- Small lesions (under 1 cm to 1.5 cm in size) or lesions with a physically small histologic complement of malignant cells: Lesions as small as 1 cm can be accurately evaluated, but the evaluation of smaller lesions may result in false negative results due to spatial resolution limitations of PET imaging and respiratory motion [7,13,21]. False negative PET scans can be seen in up to 27% of lesions 1 cm or smaller in size [67]. This error rate decreases to 10% for lesions between 1-2 cm in size [67].
Another fact to remember is that the measured SUV underestimates the true metabolic activity of a lesion when its size is less than twice the spatial resolution of the camera (which is approximately 5 mm for a PET camera) [7,13,21,66]. For a lesion 1.5 times the spatial resolution of the camera, measured maximal activity is only 60% of the true maximal activity [73]. FDG PET has shown a decreased sensitivity (80%) for malignancy for lesions smaller than 1.5 cm [41,47]. The ability to characterize smaller lesions is in part dependent on the degree of tracer accumulation within the lesion. Even very small lesions can be correctly characterized if they accumulate enough FDG to become visible. Sensitivity for lesions between 5-8 mm in size is between 50% to 73% [7,41].
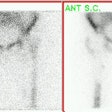
Small nodule: The patient shown below presented for evaluation of an 8 mm right lower lobe pulmonary nodule (white arrow). The PET scan was negative, but because of the lesions small size, the lack of uptake is not definitive for a benign lesion. Follow-up CT imaging will be required to document that the lesion remains stable over time. |
|
3- Hyperglycemia [47]: Competitive inhibition from high serum glucose levels appears to hinder FDG uptake in some cases. This effect is most important with acute hyperglycemia while a chronically raised glucose level inhibits tumor uptake minimally (about 10%) [41].
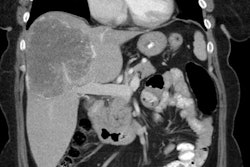
Chronic hyperglycemia: The patient below presented for staging of a left lung non-small cell lung cancer. The patient was a diabetic and had a blood glucose of 206. Because of social issues, it was elected to inject the patient and perform the scan. The images obtained were of excellent quality with marked uptake in the patients left lung mass (blue arrow). Also, several less than 1 cm lymph nodes in the aorto-pulmonary window and mediastinum were positive on the PET exam (black arrows) and these had not been called prospectively on the CT study. The patients chronic hyperglycemia likely did not significantly affect FDG tumor uptake in this case. |
|
4- Ground-glass nodules: Ground glass nodules associated with malignancy are typically bronchoalveolar cell carcinomas [73]. Low sensitivity (10%) and specificity (20%) have been reported for FDG PET imaging in the evaluation of ground glass nodules [73]. As such, there is probably little utility in the evaluation of ground glass nodules with PET imaging [73].
Based upon the present data available- a lesion that is hot on PET should be considered malignant until proven otherwise. A lesion that is PET negative has a low probability for being a malignancy (under 5% [37,66]). However, one must consider all characteristics of a lesion prior to discounting its malignant potential. Follow-up exams should be performed on PET negative nodules to ensure stability [37]. If the lesion grows, further evaluation with tissue diagnosis should be obtained [37]. Patients who have a negative FDG PET exam but are subsequently shown to have lung cancer may have an overall better survival compared to patients with positive FDG exams [7].
Nuclear medicine gamma cameras equipped with ultrahigh energy collimators have also been used to perform SPECT images of 18-FDG in patients with suspected lung cancer. Overall sensitivity of SPECT FDG is 77% to 81% for identifying malignant pulmonary lesions (sensitivity has been report to be 100% for lesions larger than 2 cm [24]). Specificity is 91-100%. Small malignancies (less than 2 cm in size) are not reliably characterized with this imaging technique (sensitivity for lesions less than 2 cm is only 20-50%). The small number of patients studied and the high incidence of malignancy in the study groups (60-75%) make any conclusions about the true sensitivity of SPECT 18-FDG premature. [10]
Nuclear medicine dual detector gamma cameras have also been modified to permit coincidence detection. In general, these cameras have a limited intrinsic detector efficiency for the high energy FDG emissions which results in poor counting statistics. Non-true coincidence detection also contributes to image noise. None-the-less, pulmonary lesions larger than 2 cm seem to be well detected due to the relatively low background activity of the lungs. Lesions within the deep mediastinum and abdomen less than 1.5 cm in size, are less well identified [19]. However, despite technical improvements lesion detectability using hybrid gamma cameras is still limited compared to dedicated PET scanners [4].
An accurate assessment of the efficacy of chemotherapy and radiation therapy would prove of enormous value in directing therapy for patients with advanced stage NSCLC [45]. On conventional imaging, a tumor response is defined as a therapy-induced reduction in the largest dimension of the tumor by 30% [73]. However, measuring the morphologic response to treatment is less than ideal as changes may take weeks to months to appreciate and therapy induced peritumoral scarring can mask tumor regression [73]. PET FDG imaging can better evaluate the effectiveness of treatment and can also provide prognostic information regarding patient outcome following initiation of therapy [29]. In fact, FDG PET exam findings may be a better predictor of survival than WHO criteria [69].
Normalization of FDG uptake (i.e.: no uptake) after treatment indicates a good response to therapy and appears to be a good prognostic indicator [41,45]. Preliminary data also indicate that patients with positive PET scans following initial lung cancer therapy have a significantly worse prognosis than patients with negative results [11,29]. In one study, all patients with negative PET exams following treatment were alive at two years (even in the presence of non-specific radiologic changes), while 50% of patients with residual hypermetabolism had died during the same two year period [41,45]. In another study, a reduction in FDG activity of 20% (by SUV analysis) after one cycle of chemotherapy was associated with a 1 year survival rate of 44%, compared to only a 10% survival rate for tumors that did not respond [69]. Further evaluation will be required to determine if changes in therapeutic regimens should be made based upon positive PET FDG results after first-line treatment. Also, a decrease in FDG uptake may only indicate a partial response to treatment resulting from destruction of sensitive cells, while resistant cells continue to be metabolically active [41,45].
Radiation therapy can result in inflammatory changes that may be difficult to differentiate from recurrent tumor [61]. This uptake is most intense in the first 6 months after therapy [61]. Generally, radiation produces a diffuse, mildly elevated FDG accumulation within the tissues included in the radiation port [41]. This activity should decrease over time and scans are more reliable when obtained at least 12 weeks after completion of XRT (although a longer wait is more desirable) [55]. Scans will be most reliable when a year or more has passed from the last radiation treatment [41]- still, uptake within the radiation port must be interpreted with caution [61]. A SUR of 2.5 still appears to be accurate in differentiating benign changes from tumor [41].
Response to treatment: 58 year old male with a left upper lobe non-small cell lung cancer and contralateral right hilar lymphadenopathy (blue arrow). FDG imaging also identified uptake in the inferolateral aspect of the right hemithorax which most likely represented a pleural metastasis with probable chest wall/rib invasion (black arrow). Because of the advanced stage of disease, the patient received radiation and chemotherapy. Follow-up PET FDG imaging demonstrated decreased tracer uptake and decreased size of the primary lesion in the left upper lobe (red arrow). The metastates in the right hilum, as well as the right pleura or chest wall have virtually resolved. Unfortunately, despite a positive response to treatment the presence of residual FDG activity in this patient would indicate a long term poor prognosis. Case courtesy of the North Texas Clinical PET Institute and CTI. |
|
Non-small cell lung cancer response to therapy: The patient shown below had widely metastatic left lower lobe lung cancer to mediastinal nodes, bones (left humerus), liver, and multiple pulmonary nodules. Note the dramatic improvement in the patient's post therapy scan (shown below). Click images to view rotating avi files. |
|
Recurrent Lung cancer: Differentiating Scar/Fibrosis from Recurrent Neoplasm:
Although no conclusive data support the use of any therapies in relapsed lung cancer, some patients with localized disease can be cured with aggressive therapy [2]. A major problem in the follow-up evaluation of patients treated for lung cancer is distinguishing post-treatment changes from recurrent tumor [48]. Presently, tumor recurrence is often not diagnosed until the disease has progressed to the point of marked enlargement of questionable abnormalities [41]. PET FDG imaging is very sensitive and highly accurate in distinguishing recurrent malignancy from scarring/fibrosis [13,41,48,49]. FDG PET imaging has been shown to have a sensitivity 98-100% for the differentiation of post-treatment change from tumor recurrence [48]. In a prospective evaluation of patients treated for NSCLC [48], FDG PET was able to correctly identify recurrent disease in all patients (sensitivity 100%) [48]. In this same group, CT imaging was non-contributory in 28% of cases at the time of FDG PET diagnosis [48].
Hicks et al also evaluated FDG PET in patients with suspected recurrent non-small cell lung cancer [2]. PET exams were positive in 41 of 42 patients with confirmed relapse (sensitivity 98%) [2]. The PET exam was negative in 14 of 17 patients with no evidence of relapse (specificity 82%), while CT was abnormal in 15 of these 17 patients (specificity 12%). Overall, CT was correct in identifying disease extent in 24% of patients, while PET was correct in 86% [2]. PET findings affected patient management in 63% of cases [2]. Patients with a positive PET exam had a poorer prognosis compared to patients with a negative study [2]. PET/CT has been shown to be superior to PET for the evaluation of suspected recurrent lung cancer secondary to improved anatomic localization of suspicious findings [64].
False positive exams can occur in association with infection and radiation pneumonitis [48]. Positive FDG PET exams due to radiation pneumonitis can be seen in up to 4% of patients receiving XRT therapy. The abnormality is usually diffuse, involves the lung and pleura, and conforms to the radiation port [2]. The duration of abnormality associated with radiation pneumonitis is variable and exams can remain positive (SUV greater than 2.5) for 6 to 15 months [48]. FDG PET findings associated with radiation pneumonitis should show decreased activity over time [48]. It is probably best not to perform FDG PET imaging earlier than 6 months after XRT in order to reduce the likelihood of false-positive PET results [48].
FDG-PET can also assist in identifying hypermetabolic foci within radiographic abnormalities to better direct tissue biopsy [45].
Screening for recurrence is another potential use of FDG PET imaging [48]. In a group of patients with NSCLC treated for cure, FDG PET screening was able to document the presence of recurrent tumor in all patients, whereas the finding was missed by CT in 44% [48]. If detected sooner, retreatment could be instituted in an effort to obtain prolonged disease control [48]. Further studies will be required to determine if improved detection of recurrent on FDG PET screening can result in improved patient survival [48].
Among patients with resected non-small cell lung cancer (NSCLC) between 30% to 50% will develop recurrent tumor [6]. The TMN staging system provides some insight into overall patient prognosis, but fails to explain differences in survival among patients with similar staged lesions. Evaluation of tumor molecular biology has provided a greater understanding of why certain tumors are more likely to recur. In patients with NSCLC, measurements of tumor proliferation estimated by proliferating cell nuclear antigen (PCNA) and Ki-67 expression have prognostic value for recurrence and survival [6]. Increased PCNA and Ki-67 expression are associated with a poor outcome [6]. FDG uptake in NSCLC has also been correlated with tumor growth rate, aggressiveness, and proliferation capacity [6]. FDG uptake has been identified as an independent prognostic factor correlated with survival in patients with lung cancer- particularly early stage lesions [6]. Varying SUV cut-offs have been determined to select patients with the greatest risk for recurrence [6]. Essentially, the higher the SUV, the higher the proliferation capacity (aggressiveness) of the tumor and the worse the prognosis [6,73].
Generalized bone marrow hypermetabolism characterized by diffuse increased axial skeletal FDG uptake has also been described in untreated patients with small cell lung cancer and is associated with an increased risk of mortality [75]. The etiology of the skeletal uptake is uncertain, but may be related to secretion of stimulating cytokines by the tumor, micrometastatic disease to the bone marrow, or host factors such as PaO2 or hemoglobin levels [75].
Radiation therapy:
FDG PET imaging can also provide important information with regards to radiation therapy planning [57]. Biologic targeting with PET can alter the planned radiation treatment volume in 30-60% of patients with non-small cell lung cancer [57]. Generally, the PET findings enlarge the planned treatment volume determined by CT alone [57] and 40% of the SUV max has been used to delineate the gross tumor volume on PET imaging [78]. However, studies indicate that PET threshold will likely need to be individualized on the basis of tumor size, location, non-uniform activity, and status of breathing control [78]. PET can also aid in demarcation of the tumor from adjacent atelectasis which will decrease the treatment portal [57]. Targeting the more metabolically active portions of the tumor with additional radiation is another benefit of the incorporation of the PET findings into the treatment plan. Long-term studies will be required to determine if the additional information provided by the PET exam results in improved patient survival [57].
Tumor motion secondary to ventilation also poses a problem for XRT of NSCLC [57]. Lower lobe lesions can move as much as 3 cm in the z-axis with respiration [57]. There are three methods to account for this tumor motion when planning therapy: 1- fuse inspiratory and expiratory CT images to incorporate the movement; 2- gate the linear accelerator to specific phases of the respiratory cycle; 3- or incorporate breath hold maneuvers during therapy [57]. With PET, the images are acquired over several minutes and thus the PET tumor volume should account for tumor excursion [57].
When performing PET for purposes of radiation planning, proper patient positioning is very important [57]. A flat table insert and use of immobilizing devices used for XRT planning aid in proper co-registration [57].
In Small Cell Lung Cancer:
Small cell lung cancer is considered to be the most aggressive form of lung cancer. It accounts for 18-25% of cases of lung cancer [73]. Small cell cancer has a high proliferation rate which results in avid FDG uptake [56]. At presentation patients are either considered to have limited disease (LD) or extensive disease (ED) [56]. Limited disease is defined as disease confined to one hemithorax and regional lymph nodes which can be targeted with a single radiation port field [56]. Disease which has extended outside the thorax or the presence of a malignant pleural effusion is considered extensive disease [56]. At presentation, about 30-40% of patients have LD, while 60-70% present with ED [56].
Treatment is based upon the extent of disease. Selected patients with very limited stage disease (T1-2 lesion and no lymph node metastatses) may be treated with surgical resection [56]. Patients with LD are treated with a combination of chemotherapy and chest radiation [56]. Patients with ED receive chemotherapy [56]. Accurate pretreatment evaluation is essential for determining optimal therapy.
PET imaging can be useful for initial tumor staging and in treatment planning for patients with presumed limited disease [70]. Preliminary work has shown that FDG PET imaging can have an impact on patient management [56]. PET imaging findings can change management in up to 37% of patients for initial staging and in up to 15% of patients for restaging [56]. PET findings can result in changes in the planned radiation field and in the detection of unsuspected sites of disease [56]. PET is also highly accurate for identifying patients in complete remission following therapy [56]. PET imaging is limited in the assessment of CNS disease in patients with small cell lung cancer [56]. MRI is the most sensitive means to exclude brain metastases [56].
REFERENCES:
(1) J Nucl Med 2001; Higashi K, et al. Comparison of [18F]FDG PET and 201Tl SPECT in evaluation of pulmonary nodules. 42: 1489-1496
(2) J Nucl Med 2001; Hicks RJ, et al. The utility of 18F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact in management and prognostic stratification. 42: 1605-1613
(3) Radiol Clin N Am 2001; Hagge RJ, et al. Positron emission tomography. Brain tumors and lung cancer. 39: 871-881
(4) Radiol Clin N Am 2001; Delbeke D, Martin WH. Positron emission tomography in oncology. 39: 883-917
(5) AJR1995; Kaiser LR, Shrager JB. Video-assisted thoracic surgery: the current state of the art. 165: 1111-1117
(6) J Nucl Med 2002; Higashi K, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. 43: 39-45
(7) Radiology 2002; Marom EM, et al. T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET. 223: 453-459
(8) Radiology 1997; 202: 435-439
(11) J Nucl Med 2003; Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. 44: 224-239
(13) RadioGraphics 1998; Frasmus JJ, et al. Thoracic FDG PET: state of the art.18: 5-20
(15) J Nucl Med 1998; 39: 234-236
(16) J Nucl Med 2003; Demura Y, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. 44: 540-548
(21) J Nucl Med 1998; Higashi K, et al. Fluorine-18-FDG PET imaging is negative in bronchoalveolar cell carcinoma. 39: 1016-1020
(24) Chest 1999; Mastin ST, et al. FDG SPECT in patients with lung masses. 115: 1012-1017
(25) J Nucl Med 1999; Coleman RE. PET in lung cancer. 40: 814-820
(29) AJR 2000; Patz EF, et al. Prognostic value of thoracic FDG PET imaging after treatment for non-small cell lung cancer. 174: 769-774
(36) Radiology 2000; Goo JM, et al. Pulmonary tuberculoma evaluated by means of FDG PET: Findings in 10 cases. 216: 117-121
(37) Radiographics 2000; Patz EF. Evaluation of focal pulmonary abnormalities with FDG PET. 20: 1182-1185 (No abstract available)
(38) N Engl J Med 2000; Pieterman RM, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. 343: 254-261
(39) AJR 2000; Boiselle PM, et al. Lung cancer detection in the 21st century: Potential contributions and challenges of emerging technologies. 175: 1215-1221 (No abstract available)
(40) Chest 2000; McCain TW, et al. The usefulness of positron emission tomography in evaluating patients for pulmonary malignancies. 118: 1610-1615
(41) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(42) Radiographics 1999; Rosado de Christenson ML, et al. Thoracic carcinoids: Radiologic-Pathologic correlation. From the archives of the AFIP. 19: 707-736
(43) AJR 1998; Erasmus JJ, et al. Evaluation of primary pulmonary carcinoid tumors using FDG PET. 170: 1369-1373
(44) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(45) Ann Thorac Surg 1998; Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. 65: 1821-29
(46) J Nucl Med 2001; Avril N, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. 42: 9-16
(47) J Clin Oncol 1998; Lowe VJ, et al. Prospective investigation of positron emission tomography in lung nodules. 16: 1075-84
(48) Eur Respir J 1999; Bury T, et al. Value of FDG-PET in detecting residual or recurrent nonsmall cell lung cancer. 14: 1376-1380
(49) J Clin Oncol 2001; Kalff V, et al. Clinical impact of 18F fluorodeoxyglucose positron emission tomography in patients with non-small-cell lung cancer: A prospective study. 19: 111-118
(50) Chest 2001; Dunagan DP, et al. Staging by positron emission tomography predicts survival in patients with non-small cell lung cancer. 119: 333-339
(51) Chest 2001; Hauber HP, et al. Positron emission tomography in the staging of small-cell lung cancer. A preliminary study. 119: 950-954
(52) J Cancer Res Clin Oncol 2000; Ilknur AK, et al. Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose in oncology: Part II: The clinical value in detecting and staging primary tumours. 126: 560-574
(53) J Nucl Med 2002; Matthies A, et al. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. 43: 871-875
(54) J Nucl Med 2002; Nehmeh SA, et al. Effect of respiratory gating on quantifying PET images of lung cancer. 43: 876-881
(55) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(56) J Nucl Med 2003; Kamel EM, et al. Whole-body 18F-FDG PET improves the management of patients with small cell lung cancer. 44: 1911-1917
(57) J Nucl Med 2004; Bradley JD, et al. Implementing biologic target volumes in radiation treatment planning for non-small cell lung cancer. 45 (Suppl): 96S-101S
(58) J Nucl Med 2004; Boucher L, et al. Respiratory gating for 3-dimensional PET of the thorax: feasibility and initial results. 45: 214-219
(59) AJR 2004; Asad S, et al. False-positive FDG positron emission tomography uptake in nonmalignant chest abnormalities. 182: 983-989
(60) AJR 2004; Cheran SK, et al. False-negative findings for primary lung tumors on FDG positron emission tomography: staging and prognostic implications. 182: 1129-1132
(61) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(62) J Nucl Med 2004; Erdi YE, et al. The C motion quantitation of lung lesions and its impact on PET-measured SUVs. 45: 1287-1292
(63) AJR 2004; Kavanagh PV, et al. Nonneoplastic diseases in the chest showing increased activity on FDG PET. 183: 1133-1141
(64) J Nucl Med 2004; Keidar Z, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. 45: 1640-1646
(65) Radiographics 2004; Lee KS, et al. T1 non-small cell lung cancer: imaging and histopathologic findings and their prognostic implications. 24: 1617-1636
(66) Radiol Clin N Am 2005; Mavi A, et al. Fluorodeoxyglucose-PET in characterizing solitary pulmonary nodules, assessing pleural diseases, and the initial staging, restaging, therapy planning, and monitoring response of lung cancer. 43: 1-21
(67) AJR 2005; Lindell RM, et al. Lung cancer screening experience: a retrospective review of PET in 22 non-small cell lung carcinomas detected on screening CT in a high-risk population. 185: 126-131
(68) Radiol Clin N Am 2005; Goldsmith SJ, et al. Radionuclide imaging of thoracic malignancies. 43: 571-588
(69) Radiology 2005; Munden RF, et al. Imaging of the patient with non-small cell lung cancer. 237: 803-818
(70) Radiographics 2006; Chong S, et al. Neuroendocrine tumors of the lung: clinical, pathologic, and imaging findings. 26: 41-58
(71) J Nucl Med 2006; Hashimoto Y, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18F-FDG uptake below the standardized uptake value of 2.5. 47: 426-431
(72) J Nucl Med 2006; Yi CA, et al. Tissue characterization of solitary pulmonary nodule: comparative study between helical dynamic CT and integrated PET/CT. 47: 443-450
(73) J Nucl Med 2006; Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. 47: 451-469
(74) AJR 2006; Shim SS, et al. Focal parenchymal lung lesions showing a potential false-positive and false-negative interpretations on integrated PET/CT. 186: 639-648
(75) J Nucl Med 2006; Prevost S, et al. Bone marrow hypermetabolism on 18F-FDG PET as a survival prognostic factor in non-small cell lung cancer. 47: 559-565
(76) J Nucl Med 2006; Shankar LK, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in national cancer institute trials. 47: 1059-1066
(77) AJR 2006; Chrietensen JA, et al. Characterization of the solitary pulmonary nodule: 18F-FDG PET versus nodule-enhancement CT. 187: 1361-1367
(78) J Nucl Med 2006; Biehl KJ, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? 47: 1808-1812
(79) J Nucl Med 2007; Kim SK, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. 48: 214-220