Prostate Cancer
Prostate cancer is the second leading cause of cancer death in
men in the U.S. after lung cancer (about 27,000 to 41,800 deaths
per year and over 200,000 new cases per year) [1,5,15,126]. The
overall lifetime risk for prostate cancer is 1 in 9 (11.1%) [121].
African-Americans have a 1.5 times greater incidence of prostate
cancer compared to whites and generally have a more advanced stage
of disease at the time of diagnosis [60]. Patients commonly
present with elevated PSA levels which prompts prostate biopsy.
However, PSA has low specificity (36%) because benign conditions
such as prostatic hypertrophy and prostatitis can also cause an
elevated PSA [35]. The specificity of PSA levels is poor below 10
ng/mL [21]. About 22-44% of biopsy proven prostate cancers
manifest with PSA levels less than 4, and 70-80% of patients with
PSA levels greater than 4 do not have prostate cancer [21]. Thus-
an increased PSA is not equivalent with a tumor and a normal PSA
does not exclude a tumor [35].
Additionally, random ultrasound guided transrectal prostate
biopsy can miss up to 50% of malignant areas found at histologic
evaluation of the prostate [9] and the false-negative cancer
detection rate is over 20% [9]. This is because the biopsy is
non-targeted and directed toward the peripheral gland (so tumors
in the anterior portion of the gland can be missed) [35]. Repeat
biopsy is necessary for patients with persistently elevated PSA
levels and negative initial biopsy [9].
The Gleason score is the most commonly used grading system for prostate cancer and provides prognostic information [17]. Because the volume of gland extracted in core biopsy is small (approximately 1% of the prostate gland) and because prostate cancer is multifocal in 85% of cases, it is not uncommon (about 30% of cases) for patients who undergo radical prostatectomy for low-grade disease to be upgraded at final pathologic analysis [35]. A clinically significant cancer has been defined as a tumor with a volume greater than 0.5 cm3 and a Gleason score of 7 or greater [60]. The commonly used D'Amico classification system defines high risk as a PSA level greater than 20, a Gleason score greater than 8, or a clinical stage T2c or T3a [83]. The risk of nodal metastases is minimal for patients with low risk disease (per d'Amico criteria); however, LN mets can be found in up to 33% of patients with intermediate- or high-risk disease [116].
Survival outcome varies considerable with the stage of the disease, with a 100% 5-year survival rate for local or regional disease and about 30% for disease with distant metastases [138]. About 78-80% of patients present with local disease, 12% with regional disease, and 4-5% with metastatic disease [25,138]. The tumor can spread via lymphatics to obturator, internal iliac, common iliac, pre-sacral, and para-aortic nodal chains [1]. Patients with Gleason grade
Prostate cancer risk stratification [138]-
| Risk Group |
Clinical & Pathologic Features |
| Very low |
T1c tumor; Tumor grade 1; PSA < 10 ng/mL; PSA density < 0.15 ng/mL/g; Fewer than 3 positive prostate biopsy fragments or cores with |
| Low |
T1-T2a tumor; Tumor grade 1; PSA < 10
ng/mL |
| Intermediate |
No high or very high risk features and one or
more intermediate risk factors: T2b-T2c tumor; Tumor grade 2-3; PSA 10-20 ng/mL |
| High |
T3a tumor or tumor grade 4 or 5 or PSA >
20 ng/mL |
| Very high |
T3b or T4 tumor or primary Gleason pattern 5
or more than 4 cores with tumor grade 4 or 5 |
Lymph node metastases:
At radical prostatectomy, nodal metastases are found in 5-10% of
patients [26,35]. Lymph node involvement is correlated with
increased risk for biochemical recurrence and progressive disease
in most patients and the five-year disease free survival rate
decreases from 85% to approximately 50% for pN1 disease [3,113].
However, survival is related to the number of involved nodes [26].
The 5-year survival rate for patients with a single nodal
metastasis is 75-80%, whereas survival decreases to 20-30% when
multiple nodes are involved [26]. The risk for nodal metastases is
related to the patients PSA level and tumor histologic grade [26].
Conventional imaging has a low sensitivity in the determination
for lymph node tumor involvement because it relies upon size
criteria [3] and 70% of metastatic nodes in prostate cancer are
small (< 8mm) [35]. For LN metastases, CT has a pooled
sensitivity of 42% and a specificity of 82% [113]. The pooled
sensitivity of MRis 39-56% and a specificity of 82-94%
[113]. A 6 mm short-axis diameter cutoff for lymph node metastases
has been reported to yield a sensitivity of 78% and a specificity
of 97% [130].
Bone metastases:
For primary staging, NCCN recommends bone scan for patients with
PSA > 20 ng/mL, a Gleason score of 8 or greater, or a clinical
stage T3 or greater (high-risk and very high risk groups) [126].
Bone scan is also recommended for patients with any two of the
following- a PSA > 10 ng/mL, a Gleason score of 7 or greater,
and clinical stage T2b/T2c (intermediate unfavorable group) [126].
A systemic review yielded rates of bone metastases of 4% for a PSA
equal or less than 10 ng/mL, 7% with a PSA of 10 to 20 ng/mL, and
42% with a PSA > 20 ng/mL [126]. Based on Gleason score, the
positive yield was 4% for Gleason of 6 or less, 10% for a Gleason
score of 7, and 29% for a Gleason score of 8 or greater [126].
For biochemical recurrence after radical prostatectomy, one study
found that patients with a positive bone scan always had a PSA of
at least 7 ng/mL and another found only 2% of bone scans were
positive for PSA up to 1 ng/mL [126]. Some suggest PSA velocity to
be an important factor to consider in these patients and the
optimal cut off being 0.5 ng/mL/month [126]. Others suggest a
trigger PSA value of 5 ng/mL and a PSA doubling time of 10 months
[126]. NCCN recommends bone scan for a PSA doubling time of 9
months or less [126].
In later stages, bone metastases can be found in over 80-90% of patients at time of death [1,130]. Bone metastases are commonly seen in the pelvis and spine (with a gradual decrease from the lumbar to cervical level), ribs, skull, and proximal ends of long bones [130]. The distribution of skeletal metastases may be in relation to areas containing hematopoietically active red bone marrow and the Baston venous plexus from deep veins to internal vertebral plexuses [130].
Treatment:
Patients with high risk cancer with a reasonable life expectancy
should be treated with prostatectomy with pelvic lymph node
dissection or radiation therapy with ADT [138]. Patients with
intermediate risk cancer who have a longer than 5 year life
expectancy are best treated with either radical prostatectomy or
radiation therapy, per patient preference [138]. Very low risk and
low risk patients can be treated with active surveillance [138].
High intensity focused ultrasound (HIFU) is a promising new
modality for the treatment of localized prostate cancer [111].
Disease control can be reached in about 81-92% of patients after
one treatment [111]. A high failure free survival of 88% has been
reported following HIFU [111]. In a small number of patients, 68Ga-PSMA
PET/MR was able to better localize tumor recurrence following HIFU
that was occult on parametric MRI [111].
Post operative adjuvant radiation therapy to the prostate bed to
eradicate residual microscopic disease is recommended for
pathologic stage pT3a/b lesions or patients with positive surgical
tumor margins [126].
See also PMSA
radioligand therapy
Biochemical recurrence:
Despite highly successful treatments for localized prostate cancer (either surgical or radiation), between 15-40% patients treated with curative intent may experience relapse within 10 years (biochemical failure with rise in PSA level) [7,25,43,51]. Following surgical prostatectomy- relapse occurs in 20-30% of patients within 10 years, while relapse can be seen in up to 53% of patients following external beam radiation within 5 years [20]. Other authors indicate a biochemical recurrence occurs in 20% of patients within 10 years of "definitive" therapy [19] and others in 20-35% of cases, with a median time to biochemical recurrence of 2-3 years after surgery [130]. The median time to develop distant metastasis after PSA recurrence without any treatment is 8 years [130].
Serum PSA should be undetectable within two months following
radical prostatectomy [51], but a PSA level up to 0.2 ng/mL
following radical prostatectomy is considered acceptable [7,24]. A
biochemical relapse following radical prostatectomy is defined as
undetectable PSA level following radical prostatectomy with two or
more subsequent PSA increases; or a serum PSA level of 0.2 ng/mL
or higher with a second confirmatory increase on a consecutive
measurement at least 3 weeks apart [73,83,130].
Following external beam radiation, the serum PSA level gradually
declines and may take 18 months or longer to reach a nadir below
which no further notable decreases occur [51]. A biochemical
recurrence after non-surgical or minimally invasive treatment
(radiation therapy or cryosurgery) is defined according to the
Houston criterion which is a PSA level 2.0 ng/mL or greater above
the nadir PSA level [24] or three consecutive increases in PSA
level after a nadir has been reached (more than 6 weeks after
therapy completion is required) [35,83]. Biochemical
recurrence does not necessarily change into cancer-specific death
[48]. One clinically important prognostic variable in these
patients is the PSA doubling time [126]. Prostate cancer-specific
survival is approximately 90% in patients with a PSA doubling time
of 15 months or greater, compared to 20% with a PSA doubling time
of less than 3 months [126].
Although rising PSA following definitive therapy suggests
recurrence, it provides no information regarding the site of
recurrent disease. About 25-35% of men with an increasing serum
PSA will develop locally recurrent disease only, 20-25% will
develop metastatic disease only, and 45-55% will develop both
local recurrence and metastatic disease [25,73]. Typical locations
for local recurrence include the perianastamotic vesicourethral
region (50-60%), retrovesicle region or seminal vesicles (10-30%),
bladder neck or base (10-20%), ureter at the vesicoureteral
junction, and stump of the vas deferens [130]. Lymph node
recurrence is considered an unfavorable prognostic factor [48].
Salvage radiation treatment (SRT) to the prostatic fossa (or
fossa + pelvic nodes in higher risk patients) is the only
potentially curative treatment option for patients with
biochemical recurrence following radical prostatectomy [82]. This
treatment is only curative if the recurrent disease is encompassed
by the irradiated volume, which are typically drawn in the absence
of radiographically visible gross disease [86]. However, many
radiation oncologists will include pelvic lymph nodes for
high-risk patients [93]. For the best chance of success, salvage
radiation therapy should be administered when the serum PSA first
reaches detectable levels, with the purpose being to treat a
disease still confined to the pelvis [41].
A patient's prognosis is improved by the initiation of salvage therapy before the PSA level exceeds 0.5 ng/mL [76]. Early salvage radiotherapy before PSA levels rise to more than 0.5 ng/mL will achieve undetectable PSA levels in more than 60% of patients [120]. It has been reported that 48% of patients who receive salvage XRT alone at PSA levels of 0.5 ng/mL or less were free of progression at 6 years, compared with 26% for those treated at higher PSA levels [41]. The overall 5-year progression free survival rate in patients that receive SRT is 56%, but varies from 71% in men with a pre-RT PSA level of less than 0.01-0.2 ng/mL, down to 18% in men with a PSA greater than 1.5 ng/mL without supplemental ADT [82]. Studies have also shown benefit (biochemical free survival, distant progression free survival) to metastatic directed stereotactic radiation treatment in the setting of oligometastatic prostate cancer (defined as three or fewer detectable metastases [130]) [126]. Nonetheless, most patients treated with salvage radiotherapy will experience disease progression after treatment [65].
In early stages, prostate cancer is a hormone-dependent disease
[142]. Therefore, patients also typically receive androgen
deprivation therapy (ADT) following any potential salvage
treatment options [74]. Studies have shown that the addition of
ADT to salvage radiotherapy can result in an approximately 20%
benefit in freedom from progression at 5 years (from 62% to 80% in
one study and 71% to 89% in another) [134]. Androgen deprivation
can be achieved by bilateral orchiectomy or with therapeutic
agents [142]. Agents such as bicalutamide, flutamide, and
nilutamide block the androgren receptor to reduce the effects of
testosterone signaling on prostate cancer cells [142]. Luteinizing
hormone releasing hormone (LHRH) agonists (leuprolide acetate,
goserelin, and triptorelin) overstimulate the pituitary to
downregulate the gonadotropin-releasing hormone (GnRH) receptor
and decrease lutenizing hormone (LH) production, which in turn
lowers testosterone production in the testes [142]. LHRH
antagonists (degarelex) block the GnRH receptor to decrease LH
production, which lowers testosterone production in the testes
[142]. Androgen pathway inhibitors (abiraterone, enzalutamide,
apalutamide, and darolutamide) target the androgen pathway to
inhibit testosterone synthesis or reduce androgen receptor
signaling [142].
However, after 2-8 years of ADT, the PSA will begin to rise
again, indicating metastatic castration-resistant prostate cancer
(CRPC- the lethal form of the disease) [74]. This resistance to
anti-hormonal therapy can be related to transdifferentiaiton of
prostate adenocarcinoma into neuroendocrine-like cells, referred
to as treatment-related neuroendocrine prostate cancer [128].
During this tumor evolution to high-grade prostate cancer,
pluripotent tumor stem cells undergo epithelial-mesenchymal
transition with increasing numbers of neuroendocrine cells that
are not regulated by androgens resulting in an acquired resistance
to antihormal therapy [128]. The prognosis for metastatic CRPC is
poor, and the median survival at this stage is 3 years [130].
Although metastatic prostate cancer may initially respond to
hormone suppression, ultimate tumor progression is inevitable [1].
Treatment in patients with metastatic CRPC includes taxane-based chemotherapy (docetaxel), the therapeutic immunostimulant vaccine sipuleucel-T, and the alpha-emitting radionuclide 223Ra [76].
Radionuclide
therapy for prostate cancer:
TNM
staging of prostate cancer
Conventional imaging in prostate cancer:
Transrectal US (TRUS): Most prostate cancers are hypoechoic
(60-80%) on TRUS, between 30-40% are isoechoic, and only 1.5% are
hyperechoic [60]. Unfortunately, only 17-57% of US evident lesions
are malignant [60]. Benign entities such as prostatitis, atrophy,
infarction, and BPH can also appear hypoechoic on TRUS [60]. For
the detection of prostate cancer, TRUS has a sensitivity and
specificity between 40-50% [60]. Lesions on TRUS are better
visualized in the peripheral zone than in the transitional zone
because of the heterogeneous pattern of the latter [60]. An
additional finding that suggests malignancy is bulging or
irregularity of the prostate capsule [60]. TRUS findings that
suggest extracapsular extension include bulge or irregularity
adjacent to a visible lesion and hypoechoic periprostatic fat
stranding [60]. Increased tumor contact (length > 23 mm) with
the capsule is also associated with a higher probability of
extracapsular extension [60]. For extracapsular extension, TRUS
has an accuracy ranging from 37-85% [60].
On color Doppler, prostate cancer typically demonstrate diffuse
increased flow compared to the surrounding prostate tissue [60].
Color imaging can be especially useful in detecting isoechoic
lesions that demonstrate increased vascularity [60].
Because prostate cancer induces neovascularity with increased
microvessel density, contrast enhanced US has been shown to be
better than gray scale imaging for the detection of prostate
cancer [60]. In a meta-analysis, CEUS had a pooled sensitivity of
70% and a specificity of 74% for the detection of prostate cancer
[60].
MRI: The combination of T2-weighted imaging and dynamic
contrast-enhanced MR imaging with endorectal coil has been shown
to have an 84-97% sensitivity and 74-89% specificity for detecting
local recurrence in the prostectomy bed [36,113]. For post
prostatectomy patients, mpMRI for the detection of local
recurrence has a pooled sensitivity of 82% and a specificity of
87%; for post radiation therapy patients with sensitivity was 82%
and the specificity 74% [113].
FDG PET in prostate cancer:
FDG PET imaging is generally not useful for the diagnosis of
primary prostate cancer primarily due to low glucose metabolic
rates and low FDG tumor uptake [6]. Urinary bladder activity also
interferes with exam interpretation [2,4]. Additionally, there is
overlap in uptake values with benign prostatic hyperplasia [6] and
false positive exams can be seen in patients with prostatitis
[25]. However, patients with higher primary tumor uptake had a
significantly worse prognosis than do patients with lower FDG
uptake [62].
A significant number of metastatic lesions from prostate cancer
will also not accumulate FDG (likely due to a low glucose
metabolic rate) [1,2,4]. FDG PET has a sensitivity about 50% for
the detection of prostate metastases (range 18-65%) [1]. Increased
tumor detection is associated with tumors with a high histologic
grade (poorly differentiated tumors with a Gleason score >7),
high serum PSA levels, and high PSA velocity [2,16,25]. The
greatest utility of PET imaging in prostate cancer may be to
evaluate changes in tumor cell burden following treatment [1] and
in patients with aggressive or hormone-refractory disease [16].
Tracer uptake in prostate cancer has been associated with a worse
prognosis with a 5-year survival of 27%, compared to 70% with FDG
negative disease [73].
For the evaluation of biochemical failure and restaging, a PSA
level of 2.4 and a PSA velocity of 1.3 ng/mL/yr provide the best
compromise for sensitivity and specificity [25]. However, other
authors suggest that FDG PET imaging has only a limited role in
patients with PSA relapse (range 0.5-40.2 ng/mL) and negative
conventional imaging [31].
Choline analogues in prostate cancer:
Choline is one of the components of phosphatidylcholine- an
essential element of phospholipids in cell membrane [3]. After
transport into the cell, choline is phosphorylated by choline
kinase to phosphocholine and trapped within the cell [44].
Malignant tumors show a high proliferation rate which results in
up-regulation of the enzyme choline kinase (which catalyzes the
phorphorylation of choline), and increased metabolism of cell
membrane components which will lead to an increased uptake of
choline [3,44]. 11C-choline is a PET tracer that can
be used for prostate cancer imaging [3]. A typical dose is 330 MBq
(which results in an effective dose of 9 mSv) [73]. 11C-choline
undergoes rapid blood clearance (about 7 minutes), rapid
metabolism to 11C-betaine, and rapid uptake in
prostate tissue [18]. Imaging can begin as early as 3-5 minutes
following tracer injection [18].
Normal 11C-choline uptake can be seen in the
lacrimal/salivary/parotid glands, liver, spleen, renal cortex,
adrenals, pancreas, and low level activity in bowel [28,50]. Low
level activity can be seen in the bone marrow (variable activity)
and in reactive inguinal and hilar lymph nodes [28,51,73]. The
agent has an advantage over FDG and 18F-choline in
that there is little urinary excretion of the agent [3,6,25],
although other authors report "substantial" bladder activity in up
to 35% of patients [11]. Urinary activity can be seen because 11C-choline
metabolites are excreted in the urine and may accumulate in the
bladder if imaging of the pelvis is not started within 5 minutes
of tracer injection [28]. Physiologic rectal activity is common
and accurate co-registration of fused images is key to detection
of abnormal tracer uptake in the surgical bed [28]. Symmetric
low-level activity in the muscular tissue of the urogenital
diaphragm is normal, as is low level activity in the penile bulb
[28]. A major limitation of the agent is it's very short
half-life (about 20 minutes) [17].
Within the prostate, the central gland typically shows more 11C-choline
uptake than the peripheral gland [28]. 11C-choline PET
can detect cancer foci in the prostate with a sensitivity of
55-81%, a specificity of 43-87%, a PPV of 71-86%, a NPV of 83-87%,
and an accuracy of 55-84% [8,11,13,25,28,31]. This is similar to
reported detection rates for MRI [31]. Lesion size has an
important influence on the exam with a sensitivity of 83% for
lesions larger than 5 mm, but only 4% for lesions < 5 mm [63].
In general, increased choline uptake in primary prostate cancer is
correlated with histologic tumor aggressiveness [22], however,
foci of high grade prostate intraepithelial neoplasm can also show
11C-choline uptake [8].
Choline uptake is not specific to prostate cancer false positive
uptake can occur in foci of acute prostatitis, BPH, and even in
normal tissue [8,28,73]. Uptake has also been described in other
neoplasms including invasive thymoma, lymphoma, renal cell
carcinoma, colon cancer, mesothelioma, lung cancer, papillary
thyroid cancer, parathyroid adenoma, and meningioma [51]. Exam
results can lead to a change in patient management 20% of cases
[63].
Lymph nodes: For the determination of lymph node metastases in a
prospective evaluation of 67 patients with histologically proven
prostate cancer 11C-choline PET had a sensitivity of
80%, a specificity of 96%, and an accuracy of 93% (compared to
conventional imaging which had a sensitivity of 47%, a specificity
of 98%, and an accuracy of 86%) [3]. Other studies have reported
sensitivities of 60-100%, specificities of 66-98%, PPV of 90%, NPV
of 87-100%, and accuracy of 88-92% [28]. For detection of
metastatic lymph nodes in patients with intermediate or high risk
prostate cancer the sensitivity has been reported to be 45-73%,
specificity 88-98%, PPV 90%, and a NPV 83-87% [14,63]. Sensitivity
is higher for nodal metastases larger than 5 mm and the agent will
fail to detect micrometastases [63].
Another benefit of PET imaging is that it can identify lymph node
metastases which are outside the field of modified lymphadenectomy
surgery [3]. False negative exams can occur in patients with
micrometastases and due to bowel activity (due the high
proliferation rate of intestinal mucosa) which may obscure a
lesion [3]. False positive exams can be seen with inflammatory
nodes [3].
Bone metastases: Active osteoblastic metastases show increased
choline activity against the normal background bone marrow
activity and rare osteolytic lesions show even higher uptake
[132]. Choline PET/CT has been shown to have better diagnostic
accuracy than bone scinitgraphy for staging high-risk patients at
diagnosis and for patients with biochemical recurrence [132].
Recurrent cancer/biochemical recurrence:
The strength of choline-based PET imaging appears to lie in the
detection of prostate cancer recurrence following prostatectomy or
radiation therapy [50]. In the evaluation of suspected tumor
recurrence in post surgical patients, 11C-choline
PET/CT has a sensitivity of 73-83%, a specificity of 88%, a PPV of
92%, a NPV of 61%, and an accuracy of 78% [28,31]. A meta-analysis
of 11C-choline PET for assessing any site of relapse
found a pooled detection rate of 62% with a pooled sensitivity of
89% and a pooled specificity of 89% [73].
For biochemical recurrence, the detection rate and sensitivity of
11C-choline increases with increasing PSA levels (about
19% detection rate when PSA is less than 1 ng/mL, compared to
about 67% detection rate when the PSA level is greater than 5
ng/mL) [20,73]. Other authors report detection rates of 36% for
PSA level below 1 ng/mL, 43% for levels between 1-2 ng/mL, 62% for
levels between 2-3 ng/mL, and 73-82% for levels of 3 ng/mL or
higher [31,73]. A retrospective analysis found positive scans in
28% of patients with a PSA below 0.5 ng/mL, in 46% with a PSA of
0.5-0.99 ng/mL, in 62% with a PSA of 1.0-1.99 ng/mL, and in 81%
with a PSA of 2.0 ng/mL or higher [133]. In this review, the
authors also noted that interpretation of choline PET scans pose
certain challenges compared to PSMA PET/CT because of relatively
high background activity, low target-to-background activity
ratios, and relatively high image noise levels [133].
Higher PSA velocities and shorter PSA doubling times may also be
associated with higher detection rates [31]. Overall, a PSA value
between 1.16 and 1.4 ng/mL is the main predictor of a positive
scan [73]. In general, for detection of relapsed prostate cancer,
11C-choline PET has a higher sensitivity compared to
FDG at all PSA levels [31]. The current recommendation is for
considering choline PET/CT as the first-line diagnostic procedure
in patients with biochemical relapse showing PSA levels greater
than 1.0 to 1.05 ng/mL, a PSA velocity higher than 1ng/mL/yr, or a
PSA doubling time less than 6 months [34,41,42]. It is important
to note that salvage radiotherapy will start at PSA levels between
0.2 and 0.3 ng/mL, at which level the detection rate for any type
of imaging will be too low for routine use [42].
For the detection of local recurrence following radical prostatectomy the reported sensitivity is 54-73%, and the specificity 88-92% [64]. The sensitivity for local recurrence is also dependent on lesion size [36]. The sensitivity for lesions less than 4mm is 20%, for 5-9mm lesions its 43%, and for lesions 10mm or larger its 82% [36]. Overall, MR is superior for the detection of small local recurrent disease [36]. In post radical prostatectomy patients, the presence of a positive 11C-choline scan carries prognostic significance with overall decreased survival compared to patients with negative scans [37].
For suspected recurrence following external beam radiation, 11C-choline
has
an
overall
sensitivity
of
81%,
PPV
of
100%,
NPV
of
44%,
and
an
accuracy
of
84%
for
the
detection
of
local,
locoregional,
and
distant
recurrence
[28].
An
focal
uptake
within
the
irradiated
prostate
should be viewed with high suspicion and help to guide biopsy
[28].
For the detection of lymph node recurrence, 11C-choline PET has been shown to be superior to MR, with a sensitivity of about 64-90% (accuracy 77-93%), compared to about 39% (accuracy 71%) for MR [36,64]. The reported specificity is 90-100%, PPV 86%, and NPV 72% [64]. 11C-choline PET can also detect recurrent metastatic disease outside of the pelvis which can be seen in up to 30% of patients [36].
In a retrospective study of prostate cancer patients with
biochemical relapse and negative bone scans, 11C-choline
PET identified 30 bone lesions in 18 of 123 patients (14.6%) [39].
Uptake in bone metastases seems to be inversely proportional to
the degree of sclerosis, although many sclerotic lesions are
tracer avid [28].
The MTV60 and the presence of extra-pelvic disease in patients
with biochemical recurrence on 11C-choline PET/CT has
been shown to be predictive of lower biochemical and clinical
relapse free survival [48].
Tracer accumulation has been noted to decrease in both the
primary tumor and in metastatic foci after hormonal therapy,
although this has been disputed in other studies [25].
The addition of choline PET to SRT planning can change the
initial plan in about 33% of patients [93].
18F-fluorocholine:
18F-fluorocholine has been developed in order to overcome difficulties associated with the short half-life of 11C-labelled compounds [7]. The distribution is similar to 11C-choline with uptake seen in the liver, spleen, kidneys, pancreas, and other exocrine organs [7]. There is variable bowel activity [31]. The agent is cleared from the blood pool within 5 minutes after administration [50]. However, 18F-fluorocholine is excreted in the urine and there is a significantly higher amount of urinary collecting system activity compared to 11C-choline [51]. Early imaging (generally within 2-10 minutes of injection [9]) and starting the exam at the level of the pelvis helps decrease the risk of significant urinary activity interfering with exam interpretation [7,51]. Tracer uptake can be seen in prostate cancer and there is no significant correlation between SUV max and the PSA levels or the Gleason score [23].
Similar to other choline agents, 18F-fluorocholine is
not useful for evaluation of the T-stage as the agent is taken up
in both prostate hyperplasia, prostatitis, and prostate cancer
[7,23]. However, dual phase imaging (early and one hour delay) may
improve detection of areas of malignancy [9]. On a dual phase
exam, malignant foci typically demonstrate stable or increasing
concentration of the tracer, while other areas will show tracer
washout (possibly related to dephosphorylation by prostate acid
phosphatase) [9].
In the preoperative staging of patients at intermediate or high
risk for extracapsular disease, 18F choline PET/CT had
a sensitivity of 66%, specificity of 96%, PPV of 82%, and NPV of
92% for the detection of lymph node metastases greater than or
equal to 5 mm (the sensitivity is limited for metastases smaller
than 5 mm) [23]. A meta-analysis of nodal stating prior to
definitive therapy found a pooled sensitivity of 49% and a
specificity of 95% [77].
The results of the 18F choline exam can change
staging in up to 33% of patients [73] and lead to a change in
therapy in 15% of patients [23]. Degenerative joint disease does
not normally demonstrate tracer uptake, but recent trauma and
fractures may take up 18F-choline [66]. The SUVmax of
18F-fluorocholine has been shown to correlate with PSA
level, pathologic stage, and post-surgical risk assessment score
(CAPRA) [69].
In the evaluation of therapy response, decreases in quantitative
parameters (SUV, MTV, total uptake) of greater than 30-35% likely
represent treatment effects [53].
In patients with elevated PSA levels and inconclusive initial TRUS biopsy results, persistent foci of tracer uptake within the prostate on dual phase imaging have been shown to correlate with areas of malignancy and can be used to help guide re-biopsy [9].
Biochemical recurrence: Biochemical recurrence is a persistent
increase in PSA following definitive treatment for prostate
cancer. Biochemical recurrence occurs in 15-77% of patients within
5-years of radical prostatectomy [33]. Ultrasound directed
transrectal biopsy is usually the first test performed in these
patients, but is only 50% effective due to false-negative results
related to sampling errors (other authors indicate positive biopsy
rates between 38% and 55% [131]) [33]. Additionally, biopsy can
only confirm local recurrence and accurate identification of all
sites of disease is critical for proper patient management [33].
In patients with isolated local recurrence, XRT has been shown to
be effective in 48-56% of patients in preventing further
recurrence for at least 3 years [33]. In patients with distant
metastatic disease, androgen deprivation therapy is the treatment
of choice [33]. However, in patients with a single metastatic
lesion, treatment may be local salvage therapy, rather than
systemic [33].
18F-choline is very useful in evaluating for
recurrent tumor following radical prostatecomy [7]. In one study,
the agent had a sensitivity of 74% for the detection of bone
metastases [16]. In another study, the agent was able to correctly
detect malignant lesions in 74% of patients [33].
The detection rate in patients with suspect biochemical
recurrence correlates with serum PSA level- 20-31% for PSA ≤ 1
ng/mL, 44% for 1 < PSA ≤ 5 ng/mL (43% for PSA between for
1-2 ng/mL [43]), and 82% for PSA > 5 ng/mL (79% for patients
with a PSA of greater than or equal to 2 ng/nL [43]) [25,43]. In
another study, the sensitivity was 77.5% for a PSA of more than
0.5, 80.7% for a PSA of more than 1.0, 85.2% for a PSA of more
than 2.0, and 92.8% for a PSA of more than 4.0 [33]. Some authors
suggest that the PSA velocity is also usually significantly higher
in patients with positive scans [31] (with a detection rate of 65%
when the PSA doubling time was 6 months or less [73]), but other
authors have not found this to be true. Some authors feel that the
exam sensitivity is degraded by the use of androgen deprivation
therapy, but other authors did not find this to be true [33].
Patients with a tumor Gleason score of greater than 7 have been
shown to have a higher detection rate for recurrence at all PSA
levels [43].
In patients with castrate resistant prostate cancer, changes in whole body tumor burden can be measured
by 18F-fluorocholine
PET/CT and estimates of metabolically active tumor burden
by 18F-fluorocholine PET/CT have
been shown to have prognostic value [55].
18F-Fluciclovine
(18F-FACBC
or Axumin):
Agent:
FACBC is a synthetic L-leucine analog that depicts amino acid
transport into cells has been approved by the FDA for detection
and localization of biochemically recurrent prostate cancer
[59,71]. The agent is a radiolabeled analog of levorotatory
leucine, which is an essential amino acid [102].
The tracer uptake is related to two different amino acid
transporters - primarily via the sodium dependent ASCT2 amino acid
transporter (alaine-serine-cysteine transporter system), but also
human L-type amino acid transporter (the large neutral amino acid
transport LAT1), and system N [59,89,102,121]. Several amino acid
transporters are up-regulated/over expressed in prostate cancer,
particularly ASCT2 in primary lesions [89,102,121]. ASCT2
over-expression has been associated with poorer prognosis and more
aggressive cell behavior [121]. LAT1 overexpression has been shown
in androgen-insensitive cell lines and it's over-expression has
also been shown to be associated with a poorer prognosis in T3 and
T4 disease [121].
Studies have found that ASCT is the dominant amino acid
transporter class for fluciclovine cellular influx and efflux in
prostate cancer, with ASCT2 being more expressed in
androgen-insensitive cell lines and ASCT1 being more expressed in
androgen-sensitive lines [121].
The agent demonstrates rapid tumor uptake,
but once inside the cell does not undergo metabolism and there
is gradual decreased avidity over time due to tracer clearance
from tumor cells via the same channels through which is entered
[71,102].
Biodistribution:
The distribution of the tracer in the body is more favorable than
choline- the uptake of FACBC in the kidneys is mild to moderate,
but negligible activity is typically found in the urinary tract
which makes the agent very useful for the evaluation of pelvic
disease (however, the bladder wall typically has physiologic
diffuse mild to moderate activity) [89,101,144]. However, urinary
activity can be seen in 5-10% of patients and this activity in the
urinary bladder can interfere with evaluation of the prostate
which typically shows mild uptake [101]. Mild urethral activity
can be seen an has a typical linear appearance [101].
A potential pitfall in the evaluation of the prostate gland is
that median lobe uptake (central base invaginating into the
urinary bladder) has a high rate of false positivity [138]. There
is prominent physiologic pancreatic and
hepatic uptake that can degrade detection of liver metastases
(the liver is also the critical organ [102]) [71,73,89]. There
is also moderate salivary/parotid and mild to moderate pituitary
uptake, variable mild to moderate esophageal (usually linear, more
prominent in the distal esophagus, and see in more than 50% of
subjects), stomach, and bowel activity, moderate bone marrow
activity (which peaks at 10-15 minutes and decreases over time),
and skeletal muscle activity that increases with time [73,102].
There is mild to moderate uptake in lymphoid tissue of the
Waldeyer ring, breast parenchyma, and the adrenal glands (which
can be unilateral or bilateral) [89]. In about 10% of patients,
adrenal uptake can be unilateral or bilateral moderate to intense
and this does not correlate with underlying pathology [101].
Mild activity can be seen in the thyroid [101,102]. Cerebral and
lung activity are minimal and below that of the blood pool [73].
The arm or subclavian vein on the side of injection may retain
tracer [89].
|
18F-Fluciclovine
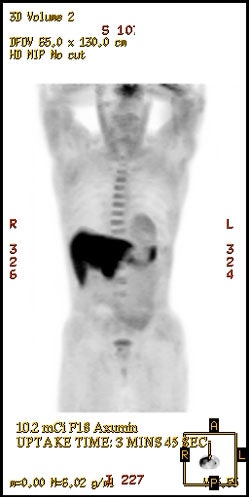
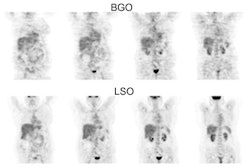
biodistribution: The image below demonstrates
physiologic tracer activity. There is also a small focus
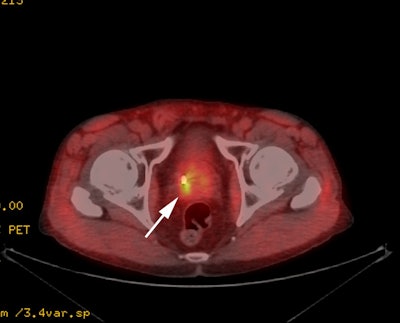
of abnormal uptake in the right aspect of the prostate.
Click image to view MIP cine. |
|
|
|
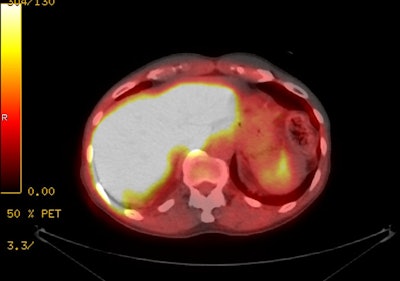
18F-Fluciclovine with
normal intense tracer activity in the liver, pancreas,
and urinary bladder. |
|
|
Nonspecific false positive uptake can be seen in BPH, infection, reactive lymph nodes, and inflammation (including post radiation inflammation) [73,89]. Uptake can also be seen in other malignancies include lung cancer, colon cancer, breast cancer, renal cell cancer, lymphoma, CNS tumors, meningiomas, multiple myeloma, and in benign osteoid osteomas [73,101,121].
Exam:
Patients should fast for 4 hours prior to the exam (except for
small amounts of water with medications) to equalize plasma amino
acid levels [89,102]. Heavy exercise should be avoided the day
before and the day of the exam because this can increase muscle
uptake of the agent [89]. Patients are also instructed not to void
for 30 minutes before the injection as it is felt that a full
bladder may decrease the amount of FACBC that is excreted into the
bladder and/or cause the dilution of any excreted radiotracer
[102].
The typical dose is 370 mBq (10 mCi) given IV in a maximum
recommended volume of 5 mL with 0.9% sodium chloride for volume
adjustment which results in an effective dose of 5.2 mSv [73,138].
There is typically rapid tracer uptake in prostate cancer with an
early peak at 5 (4-10) minutes and a plateau at 30 minutes,
followed by gradual clearance [73,102]. Imaging is typically
performed beginning 3-5 minutes following injection in a
caudocranial direction in order to optimize target-to-background
activity [73,89,102]. A 5 minute image is acquired over the
pelvis, and cranial to the pelvis, imaging is between 3-5 minutes
per bed position [102].
Exam interpretation:
Avidity equal to or greater than that of blood pool, but less
than bone marrow is considered mild; uptake equal to or greater
than bone marrow, but less than liver is considered moderate; and
uptake equal to or greater than the liver is considered intense
[102].
In prostatectomy patients, focal lesions in the prostate bed with
avidity greater than or equal to that of bone marrow are
suspicious for cancer [102]. However, if the focus of avidity is
less than 1 cm then it should be considered suspicious if the
intensity exceeds that of blood pool activity [102].
In non-prostatecomy patients, focal asymmetric or multi-focal
heterogeneous uptake in the prostate equal to or greater than bone
marrow activity is suspicious for cancer. But a small focus (<
1 cm) in a site typical for recurrence should be considered
suspicious if greater than blood pool [102]. Diffuse homogeneous
uptake is not typically pathologic unless greater in intensity
than bone marrow activity [102]. If the uptake is diffuse and
heterogeneous and greater than blood pool, it should also be
considered suspicious for disease. False positive exams can occur
due to median prostate lobe uptake (central base invagiating into
bladder) [102].
For lymph nodes, the general criterion for positive uptake in lesions larger than 1 cm is activity greater than the level of the bone marrow (preferred reference is the L3 vertebral body) in a distribution typical of metastatic prostate cancer [89,138]. For lesions small than 1 cm, positivity is suggested for activity equal to or approaching that of the bone marrow and greater than that of the abdominal aortic blood pool activity [89,138]. Nodal tracer uptake in an atypical distribution may be considered suspicious if seen in the context of other clearly metastatic disease [138]. Note that a necrotic node may not have significant uptake. Quantification may aid in nodal classification with a node SUVmax to L3 marrow SUVmean ration of >/= 1.2, as a threshold. Mild symmetric physiologic uptake can be seen in external iliac, hilar, and axillary ndoes and these sites are atypical for metastatic disease [102].
Metastatic bone lesions can demonstrate tracer uptake, even when the corresponding CT exam appears negative. Focal bone uptake that is clearly visualized on MIP or PET images can be considered suspicious for cancer [102]. A sclerotic bone lesion on CT without tracer uptake, however, does not exclude a metastasis as sclerotic lesions can be negative and correlation with bone scan, F-18 NaF imaging, or MRI is recommended. Tracer uptake in lytic lesions tends to be intense and moderate in mixed lesions [102]. Degenerative uptake in bone is not as common as see with FDG, but may be mild in intensity [102].
Non-prostate lesions- Any focal uptake associated with a renal
mass in the kidney should be considered suspicious for malignancy
[102]. Adrenal adenomas may demonstrate mild to moderate tracer
uptake [102]. Primary brain tumors and brain metastases have
variable uptake that is usually greater than that of the brain
parenchyma [102]. Infectious and inflammatory processes may also
result in increased tracer uptake and mild to moderate uptake can
be seen at sites of subcutaneous injections [102].
Results:
For the detection of primary lesions, the reported sensitivity is
between 81-92.5% and specificity is 50-90%, likely due to overlap
of tracer uptake in BPH [89]. Combined FACBC/MRI imaging has been
shown to improve the PPV from both exams [89,90].
In primary staging, the agent has been shown to have a low
sensitivity for the detection of LN metastases and exam results
should not replace lymph node dissection [89].
The reported limitations of the agent are uptake in other
malignancies, intense physiologic uptake in the liver and
pancreas, urinary excretion, and low uptake in sclerotic bone
metastases [131].
Recurrent disease:
The agent has been used for the detection of recurrent prostate
cancer [71]. Up to 34% of patients with biochemical recurrence and
metastatic disease can have a single metastatic lesion [149]. The
overall detection rate is between 37-68% [73] and a positive
predictive value of 72% for local recurrence and 92% for
extraprostatic recurrence [131]. Exam results are affected by the
PSA level with higher detection rates reported for higher PSA
levels, shorter PSA doubling times, and patients with higher
original Gleason scores (greater than 7) [73,89,112,130,138].
Performance for the detection of local recurrence has also been
shown to be better in patients that have undergone prior
prostatectomy than in those with prostate sparing therapies [131].
In one study, the reported detection rate was 41% for patients
with PSA levels of 0.79 or less [73]. In another study, there was
a 31% rate of inaccurate interpretations in patients with PSA
levels ≤ 1 ng/mL, and a 16% inaccurate rate in patients with PSA
levels > 1 ng/mL [56]. Other studies report detection rates of
21-72% for a PSA of less than 1 ng/mL, 29% to 83% for PSA between
1 to < 2, 45% for PSA 2 to < 3, and 59% for PSA greater than
3 [89,126,130]. Some authors have concluded that overall, 18F-fluciclovine
is less likely to yield a positive result in patients with a PSA
lower than 1ng/ml, unless the doubling time is rapid [102].
However, another retrospective study reported a detection rate of
58% for patients with a PSA of less than 1ng/mL and that
extraprostatic disease was identified in 38% of these patients
[112]. Lesion detection was 87% for patients with a PSA level of 1
to less than 2, and 96% for patients with a PSA of 2 or higher
[112]. These authors also found no decreased detectability in
patients receiving androgen suppression [112]. Another study found
that the agent was probably not useful for the detection of
recurrent disease in patients with very low PSA levels (less than
0.3 ng/mL), but that that the agent may be useful for PSA levels
between 0.3-1 ng/mL [144]. It has also been suggested that for
patients with PSA levels less than 1.0 ng/mL, the detection rate
for abnormalities is much higher in post prostatectomy patients
compared to those who have not had surgery [144]. Prostate MRI can
be used to further evaluate equivocal findings in the prostate
gland [144].
|
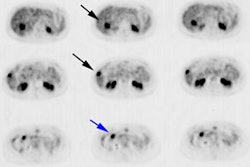
18F-Fluciclovine for
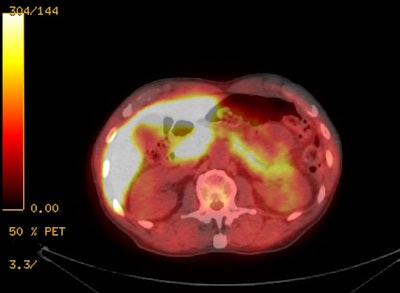
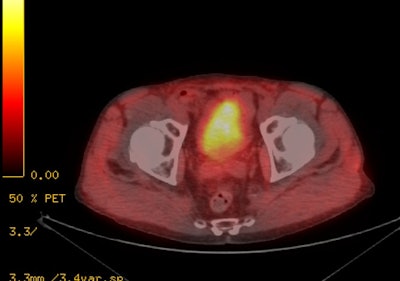
biochemical recurrence: The patient below had
undergone prior external XRT for treatment of prostate
cancer and had suspicion for biochemical recurrence based
on slowly rising PSA. The exam demonstrated focal uptake
in the right prostate adjacent to an internal fudicial
marker. The finding is seen on both AC (second image) and
non-AC images (third image) which confirms the finding is
not an artifact related to the beam hardening associated
with the marker. The referring physician elected not to
pursue the exam finding and was primarily concerned with
the presence of nodal disease which was not evident on the
study. |
|
|
Note- a recent article in a small number of patients suggested that 18F-fluciclovine PET is markedly inferior to 68Ga-PSMA PET imaging in patients with suspected recurrence, with false negative exams and underestimation of disease extent [91].
For the detection of bone metastases, the agent typically
demonstrates intense uptake in lytic bone lesions, moderate uptake
in mixed sclerotic lesions, and little to no uptake in dense
sclerotic lesions [89].
Impact on patient management:
Management can be changed in 40-59% of patients based on the exam
results [73,112,126].
Impact on prognosis:
18F-fluciclovine PET exam results can influence
failure free survival [149]. In a prospective trial of patients
with biochemical recurrence comparing salvage XRT using standard
imaging to standard imaging plus 18F-fluciclovine PET,
the 3-year failure-free survival was 63% for standard imaging and
75.5% for standard imaging plus 18F-fluciclovine PET
[149]. At 4 years, the failure-free survival was 51.2% for
standard imaging and 75.5% for the group with the addition of 18F-fluciclovine
PET [149].
Despite these encouraging results, treatment failures do occur outside of the SABR field because of the fundamental limited sensitivity for the detection of micrometastases that go unrecognized
11C-acetate:
11C-acetate has been studied in the evaluation of
suspected recurrent prostate cancer [4]. Acetate enters the
biochemical pathways of fatty acid metabolism (which is
upregulated in prostate cancer) [32]. The cellular retention of
radiolabeled acetate in prostate cancer cell line is primarily due
to incorporation of the radiocarbon into phosphatidylcholine and
neutral lipids of the cells (it is an indirect biomarker of fatty
acid synthesis) [25,29]. The upregulation of fatty acid synthetase
(FAS) may play a role in 11C-acetate uptake in
prostate cancers (11C-acetate serves as a biomarker of
FAS activity) [15,50,62]. FAS is a multifuncational enzymatic
protein that catalyzes fatty acid biosynthesis- it is
overexpressed in prostate cancers (as well as other tumors), but
levels are low or absent in most normal tissues [15].
Over-expression of FAS correlates with prostate cancer
aggressiveness and high Gleason scores [62]. However, fatty acid
synthesis expression does not appear to fully explain tracer
uptake in prostate cancers [29].
There is physiologic widespread tracer uptake by the
heart/myocardium, kidneys, liver, salivary glands, pancreas,
spleen, intestine, bone marrow, and skeletal muscle [50]. 11C-acetate
gets metabolized to 11C-CO2 in normal
tissues and this is in turn eliminated by way of the lungs [50].
There is very little urinary excretion of the agent (because of
active reabsorption of 11C-acetate in the proximal
convoluted tubule) which is useful for imaging prostate cancer
[25,32,50,62]. Because of the rapid uptake and metabolism of the
agent, PET images are typically acquired between 5 and 15 minutes
after tracer administration [50]. The agent is accumulated in
prostate cancer and can detect more sites of disease when compared
to FDG imaging [4]. 11C-acetate uptake has been shown
to be higher than FDG in local recurrence and regional lymph node
metastases, while FDG uptake is higher in distant metastases [25].
The effective whole body dose is low at 0.0049 mSv/MBq [62].
Primary tumor: The success rate for lesion detection has been
shown to correlate with PSA levels [25] and tumor size [29].
Positive exam findings can be found in up to 59% of patients with
PSA values greater than 3, however, in this study not all sites of
uptake were histologically proven to correlate with metastatic or
residual disease [4]. Reported sensitivities range from 59-83%,
and a specificity of 80% [14,29]. One drawback of 11C-acetate
imaging is that uptake can also be seen in the normal prostate
gland and in areas of benign prostatic hyperplasia which can be
similar in intensity to prostate cancer and can result in a large
number of false-positive findings [5,6,29,62]. Tracer uptake can
also occur in prostatitis [30] and other cancers [50].
Metastatic disease: In one study of intermediate or high risk
patients with negative conventional imaging studies, 11C-acetate
PET/CT detected pelvic lymph node or distant metastases in 34% of
patients [32]. The per-patient sensitivity for detection of lymph
node metastases has been reported to be variable between 38-68%,
specificity 78-96%, PPV 49%, and NPV 89% [32,62]. A meta-analysis
of 11C-acetate for regional lymph node metastases
indicated a sensitivity of 73% and a specificity of 79% [50].
Prognosis/Management: Treatment free survival has been shown to
be worse in PET-positive patients- even in those patients with
presumed "false-negative" results (suggesting that there may have
been lack of proper pathologic identification of tumor involved
lymph nodes) [32]. By 1 year, 79% of patients with positive PET/CT
exams had experienced treatment failure compared to 26% of
patients with negative PET/CT exams [32]. PET findings can affect
patient management by changing the radiation approach in more than
40% of patients [62].
Recurrent disease: For the detection of local recurrence in the
prostate fossa, 11C-acetate PET/CT has a reported
pooled sensitivity of 83%and a specificity of 92% [50]. For the
detection of recurrence in regional lymph nodes the agent has a
pooled sensitivity and specificity of 82% and 94%, respectively
[50]. The rate of positive exams increases with serum PSA levels
and PSA velocity (threshold of PSA velocity of 1.32 ng/mL/yr and a
PSA level of 1.24 ng/mL [62].
11C-methionine:
11C-methionine can identify more lesions than FDG (sensitivity about 72% compared to conventional imaging modalities) [1]. 11C-methionine accumulation in tumor cells is attributed to increased amino acid transport and metabolism in tumor cells [1]. The agent is metabolized in the liver and pancreas and has minimal renal excretion [16].
Anti-1-amino-3- 18F - fluorocyclobutane-1-carboxylic
acid (anti-FACBC) is a synthetic L-leucine analog that has been
shown to be taken up by prostate tumors [18]. It's bladder
excretion is low [18].
PSMA agents:
PSMA is a membrane gyloprotein which is up-regulated in castrate-resistant and metastatic prostate cancer [46]. Its expression is 100 to 1000-fold higher in prostate cancer than in other tissues and PMSA expression levels increase with higher tumor stage and grade (higher Gleason score) [50,74,85]. The degree of PMSA expression is also associated with the time to tumor progression and the probability of cancer relapse [50]. Between 5-10% of primary prostate cancer lesions are PMSA-negative [74]. Poorly differentiated prostate cancer with neuroendocrine differentiation is a subtype of prostate cancer may appear negative at PSMA-directed imaging [50]. Androgens will down-regulate the expression of PMSA and it is more abundant on the surface of castration-resistant tumors [50].
PMSA is not specific to the prostate gland and is expressed in
other normal tissues (salivary glands, duodenal mucosa, jejunal
brush border, subset of proximal renal tubular cells, and
sub-population of neuroendocrine cells in the colon crypts), as
well as certain neoplasms (subtypes of transitional cell
carcinoma, renal cell carcinoma, salivary gland ductal carcinoma,
lung cancer (pulmonary adenocarincoma), hepatocellular carinoma,
glioblastoma multiforme, thyroid cancer, and colon carcinoma)
[46,74,83,85]. However, the overall detection rate of other
malignancies on 68Ga-labeled PMSA imaging has been
suggested to be very low (0.7%) and that atypical foci of tracer
uptake are more likely to represent unusual prostate cancer
metastases [78]. Tracer uptake has also been described in Paget
disease, fractures, vertebral body hemangiomas, thyroid adenoma,
adrenal adenoma, and in granulomatous disease [74,75].
68Ga-PSMA-11:
The small molecule agent 68Ga-PSMA-11 has an
advantage over antibody agents by achieving better tumor
penetration and faster clearance from the blood pool [85]. The
agent binds to the active site of the PMSA molecule [85]. The
biodistribution of 68Ga-PSMA includes physiologic
uptake in the lacrimal and salivary glands, parotid glands, liver,
spleen, kidneys, and intestine/colon [73,74]. High physiologic
activity in the liver may potentially obscure liver metastases
[73]. Unbound 68Ga-PSMA tracer is filtered at the
glomerulus and excreted by the kidneys into the urinary bladder
[73,85]. Physiologic uptake is also seen in nervous system ganglia
such as the celiac ganglia located in front of the crura in a
paraaortic location and in the cervicothoracic ganglion which lies
anterior to the transverse process of the C7 vertebral body and
this should not be misinterpreted as metastatic disease [73,74].
Marrow activity is typically lower than that seen with choline
agents [73].
Examination: The dose is 1.8-2.2 MBq/kg IV, and a typical dose of
150 MBq results in an effective dose of 2.4 to 5 mSv [73,83].
Lower doses have been shown to be associated with a negative
impact on image quality and lesion detectability [119]. Patients
should be well hydrated to help reduce artifacts from high tracer
activity in the urinary system [74]. Imaging is typically
performed 1 hour (45-75 minutes) following tracer injection [73],
but delayed imaging at 3 hours has demonstrated higher uptake and
decreased background activity resulting in detection of a higher
number of lesions [74,145]. Patients should be well hydrated and
void immediately prior to initiation of imaging [83]. PET images
should be acquired from the pelvis to the head due to decrease
urinary activity in the bladder that can interfere with evaluation
of the prostate [83].
Normal distribution:
The highest physiologic activity is seen in the kidneys (eight
times the level of hepatic uptake), ureters, and urinary bladder
due to renal excretion [83,110]. There is high physiologic
activity in the lacrimal, parotid (three times hepatic activity),
and submandibular glands [83,110]. The agent is secreted in the
saliva and oropharyngeal, laryngeal, or esophageal activity can be
seen [83]. High intensity uptake may be seen in small bowel,
primarily in the duodenum [83] and also in the descending colon
and rectum [110]. Moderate intensity activity is seen in the liver
and spleen, and low level activity in the blood pool [83].
Activity can also be seen in Waldeyer's ring in the neck and vocal
cords [110].
Low PMSA uptake is also seen in parasympathetic ganglia, most
commonly the celiac and stellate ganglia, but can also be seen in
the presacral ganglia [83]. The uptake is typically low intensity
and linear or comma shaped [83].
Low-to-moderate PMSA uptake can be seen in association with osteoblastic activity associated with osteoarthritis, degenerative change, and fractures [83]. Low-to-moderate uptake can also be seen in fibrous dysplasia and Paget disease [83]. Prominent uptake, however, is seen in cutaneous, vertebral, and hepatic hemangiomas [110]. Increased uptake is also seen in acute and chronic inflammation, such as synovitis [110]. Tracer uptake can also been seen in multiple other benign and malignant lesions [110].
Another potential drawback for 68Ga-labeled tracers is resolution due to positron range effects [70]. Additionally, there can be low or absent PMSA expression on visceral metastases [74].
Image interpretation:
A scoring system has been suggested to aid in exam interpretation
[110]-
0- Uptake less than that of blood pool
1- Low uptake equal to or greater than that of blood pool, but
less than the liver
2- Intermediate uptake equal to or greater than the liver, but
less than parotid gland
3- High uptake equal to or greater than the parotid gland
Primary tumor:
68Ga-PSMA localization in the prostate has good
correlation with location and extent of prostate cancer [52,67].
There is also a positive correlation between lesion tracer uptake
and higher Gleason scores (greater than 7) and higher serum PSA
levels (10 ng/mL or greater) [79,87,110]. In one histopathologic
study, using an SUVmax of 6.5 or greater, the agent had a
sensitivity of 67%, specificity of 92%, PPV of 97%, NPV of 42%,
and an accuracy of 72% for localizing tumor involved segments
[67]. In another study, an SUVmax of greater than 6.9 was
suggestive of an overall tumor Gleason pattern of at least 7 [97].
Other authors indicate that an SUVmax of 3.15 or greater can be
associated with a sensitivity of 97% and a specificity of 90%
[87].
In a meta-analysis of 68Ga-PMSA PET/CT
for initial detection of prostate cancer (mean PSA level for
the entire cohort was 12.9 ng/mL), the agent had an overall
sensitivity of 97% and a specificity of 66% [148]. In
one study, 68Ga-PSMA was shown to be superior to MR in
detection of primary prostate lesions (MR has a reported overall
sensitivity of 48-78%) [96]. However, combined 68Ga-PSMA
PET/MRI has been shown to outperform multi-parametric prostate MRI
and 68Ga-PSMA PET for tumor localization on a sextant
basis [74,97]. Despite the high diagnostic accuracy, a drawback is
the agents moderate specificity due to tracer uptake in other
conditions such as prostatitis, granulomatous disease, and BPH
[148].
False-negative findings are most often associated with prostate
cancer segments with a Gleason score of 7 or less, a segmental
tumor burden of less than 25%, and in patients with a PSA level of
less than 10 ng/mL, compared to patients with a PSA level of 10 or
more [67,73]. SUVmax has been shown to correlate with the PSA
level [96]. About 5-10% of prostate cancers do not demonstrate
appreciable 68Ga-PSMA uptake [73].
Dual phase imaging (early 5-7 minute and late 50-60 minute
imaging) can improve the accuracy of classifying malignant versus
benign prostate lesions [94]. There is increased concentration of
tracer (and SUVmax) in malignant lesions on delayed images, while
benign lesions show no significant difference in tracer
concentration on early versus late phase images [94]. However, a
minority of malignant lesions may demonstrate a lower or stable
SUV on late phase imaging [96].
In initial staging, PMSA PET has a pooled sensitivity of 74% and
a specificity of 96%, using nodal pathology at prostatectomy as
the reference standard [149]. PMSA PET imaging findings can result
in a change in TNM stage in up to 26% of patients, a change in
management in 21% of patients, and a change in planned
radiotherapy in 36-44% of patients [99,106,108].
Nodal disease:
Conventional imaging using size criteria is limited for the
accurate detection of lymph node metastases [107]. Nodes outside
the true pelvis are classified as nonregional nodes and designated
M1a- inguinal, common iliac, paraaortic, aortocaval, and
retrocrural nodes [83]. For detection of nodal involvement in
intermediate to high-risk patients, the agent has been shown to be
superior to CT/MR with a sensitivity of 66% (vs 39-44% for CT/MR)
and a specificity of 99% (vs 82-85% for CT/MR) [73,107]. Overall
reported sensitivity for detection of nodal mets has been reported
to be 50-67% [96]. For initial staging, a metaanalysis using nodal
pathology at prostatectomy as a gold standard, found 68Ga-PSMA-11
had an overall sensitivity of 74% and a specificity of 96% [103].
One study suggested that 68Ga-PMSA PET was superior to
18F-choline
for the detection of lymph node metastases due to detection
of smaller metastatic lesions [107,113].
In one study using PMSA PET CT for staging newly diagnosed
prostate cancer (with a large portion of high risk patients - 84%)
PET positive nodes were found in 32% of patients [116]. Most nodal
metastases occurred in the pelvis, but 36% of nodal metastases
were in extra-pelvic sites and 3% of patients demonstrated Virchow
nodes [116].
In another study of newly diagnosed high risk patients advanced
disease (N1/M1) was observed in 35% of patients and was associated
with increasing PSA level, clinical stage, and ISUP grade [147].
However, metastatic disease was still identified in 18.5% of
patients with a PSA level of less than 10 ng/mL [147]. Metastatic
disease frequency was 11% for ISUP grade 2 patients (Gleason score
3+4) versus 37% for ISUP grade 3 patients (Gleason score
4+3) [147]. The overall sensitivity for lymph node
metastases was 31%, specificity 96.5%, PPV 69%, NPV 84.5%, and
accuracy 83% [147]. Undetected lymph node metastases
micrometastases or without PMSA expression [147]. Bone
metastases were present in 17% of patients (in 22.5% as the
only site of metastatic disease and with concurrent LN mets
in the other 77.5% of BM mets patients) [147]. BM mets were
identified in 8% of high risk patients with a PSA of less
than 10 ng/mL and in 11.3% of patients with PSA levels
between 10-20 ng/mL [147]. A separate study of newly
diagnosed prostate cancer patients found BM mets in 3.2% of
patients with a PSA of less than 10 ng/mL and in 9.6% of
patients with a PSA between 10-20 ng/mL [147].
False-positive findings have been reported in association with
the cervical, celiac, and sacral ganglia, but uptake in these
regions is typically relatively low [80].
Distant metastatic disease:
The most common site of extranodal disease is the bone (M1b disease) [83]. The agent has been shown to be superior to planar bone scintigraphy for the detection of bone metastases [73]. In one study, 68Ga-PSMA imaging found bone metastases at initial staging in 17% of patients with PSA levels below 5 ng/mL [123].
Visceral metastatic disease is designated M1c and PMSA PET is
very sensitive for detection of visceral mets [83].
Biochemical recurrence:
The agent can also be used for the evaluation of patients with
suspected biochemical recurrent prostate cancer [46]. Conventional imaging
underestimates lymph node disease in patients with biochemical
recurrence [80]. Only 11-14% of patients with biochemical
failure after radical prostatectomy have positive CT findings
[80]. 68Ga-PSMA PET CT can identify disease
outside of the prostate bed in 28-43% of men with rising PSA
following radical prostatectomy [82]. In one study, only 48% of
all 68Ga-PSMA PET positive nodes were pathologic by
size criteria on CT [80].
For detection of biochemical recurrence, a
metaanalysis, using pathology as a gold standard, found 68Ga-PSMA-11
had a detection rate of 63% when the PSA was less than 2, and
94% when the PSA was greater than 2 [103]. As with other agents,
the higher the PSA level (or higher the PSA velocity), the
greater the likelihood for a positive PMSA exam (up to 88% for a
PSA greater than 2 ng/mL) [47,84]. A
meta-analysis reported a pooled detection rate of 75% in
patients with biochemical recurrence [73]. Another meta-analysis
found a per-lesion sensitivity of 80% and a specificity of 97%
[80]. In another meta-analysis, the
reported detection rate was 48% for a PSA of 0.2 ng/mL, 56% for
a PSA of 0.5, and 70% for a PSA of 1.0 [83]. A retrospective
study of patients with biochemical recurrence following radical
prostatectomy found detection rates of 58% for PSA 0.2 to <
0.5 ng/mL, 73% for PSA 0.5 to < 1.0, 93% for PSA 1 to <
2.0, and 97% for PSA > 2 ng/mL[130]. Other authors indicate a
positive scan can be seen in 60% of patients with a PSA between
0.05-0.5 ng/mL and in 80% of patients with a PSA between 0.5-1.0
ng/mL [82]. However, a prospective study found detection rates
of 32% for PSA < 0.2 ng/mL, 45% for between 0.2 and < 5
ng/mL, 71% for a PSA between 0.5-1.0 ng/mL [145]. Decreased
detection rates at lower PSA levels may be related to urinary
excretion and intense urinary bladder activity that may obscure
small locoregional lesions [145].
In another study of patients with suspected
recurrence following primary radiation therapy, the agent
detected recurrence in 91% of patients [72]. The detection rate
was dependent on PSA with a detection efficacy of 82% for PSA 2
to < 5 ng/mL, 95% for a PSA of 5 to < 10 ng/mL, and 97%
for PSA of greater than or equal to 10 ng/mL [72]. Another study
found a positive exam rate of 64% for PSA doubling times of
greater than 6 months and 92% for a doubling time of less than 6
months [84]. However, lower detection rates have been reported-
16% for PSA 0.2- < 0.5 ng/mL and 20% for PSA 1- < 2 ng/mL,
but this may be related to exclusion of patients on androgen
deprivation therapy [84].
68Ga-PSMA PET MRI has also
been shown to have a high detection rate for recurrent prostate
cancer even at low PSA levels [120]. In one study of
post-prostatectomy patients, the detection rate was 65% in
patients with a PSA between 0.2 and 0.5 ng/mL and 38.5% in
patients with a PSA of less than 0.2 ng/mL [120]. For
post-prostatectomy patients this same article reported prior 68Ga-PSMA
PET CT studies have demonstrated detections rates of 38-55% for
PSA levels of 0.2-0.5 ng/mL [120]. The article also noted that
despite PSA values 0.5 ng/mL or less, approximately 39% of
patients with positive exams were found to have disease outside of
the standard salvage radiotherapy volume [120]. The most common
site for PMSA recurrence outside of the prostate bed was lymph
nodes, however, on 13% of the PSMA positive nodes were larger than
8 mm (previous studies have noted only 36% of lymph nodes detected
on PSMA PET are enlarged by size criteria) [120].
68Ga-PSMA PET has been shown to be superior to
11C-choline and 18F-fluciclovine PET in depicting recurrent
prostate cancer, especially when the PSA level is less than 1ng/mL
[130]. 68Ga-PSMA has also been shown to have a
significantly higher detection rate than 18F-fluoromethylcholine,
particularly for patients with low PSA levels [47,83]. In
patients with a PSA level below 0.5 ng/mL, PMSA imaging can be
positive in up to 50% of cases, compared to 12.5% for fluoromethylcholine [47]. In another prospective
study comparing PMSA PET/CT to 18F-choline PET/CT
the detection rate was 66% for PSMA versus 32% for 18F-choline [83].
False negative exams can be seen in
association with small volume disease (< 4mm) and due to
obscuration of prostate fossa disease by excreted activity in
the urinary bladder [82,83].
Therapy response:
68Ga-PSMA-11 uptake can be affected by androgen
blockade therapy (due to changes in PMSA expression), the effect
occurs early after commencing treatment, and the effect is
dependent on the castration sensitivity of the cancer cells at the
time of imaging (castration sensitive versus castration resistant)
[104,143]. A rapid reduction in 68Ga-PSMA-11 PET
intensity has been noted in men with hormone-sensitive disease
(castration sensitive prostate cancer) that commence androgen
blockade treatment and PMSA-PET may significantly underestimate
the volume of metastatic disease in hormone-naive men who have
commenced treatment even within 9 days [104]. Patients
with metastatic CSPC showed a median 30% reduction in SUVmax
from baseline following ADT therapy [143]. Accurate
staging with PMSA PET should therefore occur before beginning
androgen blockade treatment [104].
In contrast, men with metastatic castration-resistant prostate cancer starting ADT therapy have been shown to demonstrate an increase in 68Ga-PSMA-11 PET intensity by day 9 [104]. Patients with metastatic CRPC demonstrated a median 45% increase in SUVmax [143].
Other authors report there can be an increase in intensity of tracer uptake early after commencing hormone-deprivation therapy, which can result in a "flare" phenomenon on PMSA PET imaging [83]. This may be related to short-duration ADT increasing PSMA expression in metastatic lesions, with 59% more lesions imaged with 68Ga-PSMA PET, reminiscent of the flare phenomenon that can be observed with bone scintigraphy after ADT administration [110]. In contrast, continuous long-term ADT use (median duration 230 days) by patients with castrate-suspectible prostate cancer was associated with detection of 55% fewer lesions (reduced depiction of castration-sensitive prostate cancer lesions) [110,142].Efforts are underway to define therapy response assessment using PMSA imaging agents [143].
Prognosis: 68Ga-PMSA imaging can lead to
reclassification of stage in up to 61% of patients with
biochemical relapse (from cN0 to cN1) [80] and have a high impact
on patient management (62% of patients) [134].
Importantly, in patients with disease confined to the prostate
fossa on 68Ga-PSMA, 81% responded to SRT (compared to
53% if the scan was positive for lymph nodes or distant
metastases) [82]. A negative PMSA scan was
also associated with a very good 86% response rate to pelvic
salvage RT [82]. Another prospective study found that PMSA PET
exam findings were highly predictive of freedom from progression
at 3 years following salvage RT - FFP was seen in 81% of
patients with negative or prostate fossa confined disease at
PMSA PET versus 45% in patients with extra-fossa disease [134].
Patient management:
PSMA PET has been shown to have a high impact
on patient management [83]. Findings on 68Ga-PSMA
imaging can affect patient management in 34% to 63% of cases
[47,81,84,99,106,108,115]. In a prospective study, 68Ga-PSMA
exam results led to a change in management in 50-62% of patients
with biochemical recurrence and in 21% of patients during primary
staging [84,88]. Even in patients with low PSA levels with
suspected biochemical recurrence (PSA < 1.0 ng/mL) 68Ga-PSMA
could have a major impact on SRT in up to 19% of patients and a
minor impact in 30% [86]. The changes typically involved extending
planned radiation therapy fields to include regional nodes or
resection of regional nodes during surgery [84]. More importantly,
studies have demonstrated similar recurrence free survival rates
for PET positive and PET negative patients with biochemical
recurrence, suggesting that treatment intensification based on PET
exam findings leads to improved patient outcomes [98].
However, treatment failures do occur outside of the SABR field because of the fundamental limited sensitivity for the detection of micrometastases that go unrecognized [149]. In field disease control is generally very good, but recurrence outside the radiation field can be seen in 46-75% of patients [149].
PMSA imaging can also play a role in determining patient
eligibility for 177Lu-PMSA
radioligand therapy [83].
Comparison to other agents:
68Ga-PSMA-11, 18F-Fluciclovine, and 11C-choline
all have similar detection rates in biochemical recurrence for
PSA levels > 2 ng/mL [131]. 68Ga-PSMA-11
and 18F-Fluciclovine
appear superior to 11C-choline
at PSA levels between 1-2 ng/mL [131]. 68Ga-PSMA-11
has superior performance to the other two agents at PSA <
1ng/mL [131].
A meta-analysis comparing PMSA imaging to 18F-Fluciclovine
found pooled detection rates of [136]:
PSA < 0.5 ng/mL: PMSA agents- 45%; 18F-Fluciclovine- 37%
PSA 0.5-0.9 ng/mL: PMSA agents- 59%;
18F-Fluciclovine- 48%
PSA 1.0-1.9 ng/mL: PMSA agents- 80%;
18F-Fluciclovine- 62%
A prospective study comparing 68Ga-PMSA and 18F-Fluciclovine in the setting of
early biochemical recurrence following prostatectomy (PSA
0.2-2.0 ng/mL) also found a significantly higher per-patient and
per-lesion detection rate with PMSA compared to Fluciclovine [137].
18F-PSMA:
The
advantage of 18F
labelled agents versus 68Ga is their
longer half life (110 vs 68 minutes)
and their lower positron energy that improves spatial
resolution [100]. 18F-PSMA exhibits
rapid blood clearance and has only minimal activity (1-2%
during the first 2 hours) excreted via the urine (18F-PSMA-1007
is excreted primarily through the liver) which
aids in detection of local recurrence [100,124,129,146]. In
a direct comparison, 18F-PSMA-1007
was shown to be superior to 68Ga-PMSA-11
PET/CT for detection of lesions close to the urinary
bladder and ureter [146].
In
one study for initial staging, on a patient based
analysis for detecting lymph nodes larger than 3 mm, 18F-PSMA-1007
had a sensitivity of 86% and a specificity of 99.5%
[146]. The patient based sensitivity for detecting
lymph nodes of any size was 73.5% and the specificity
was 99.4% [146]. On a per lesion basis for lymph nodes
more than 3mm, the sensitivity was 64%, the
specificity 91%, the PPV 75%, and the NPV 86% [146].
In
patients with biochemical recurrence, an initial study of 18F-PSMA
demonstrated disease detection in 81% of patients [100]. In
another study of a small number of patients (9 patients)
with biochemical recurrence, for lymph
nodes larger than 3 mm, 18F-PSMA-1007 had a
sensitivity of 79%, a specificity of 100%, a PPV of
100%, and an NPV of 98% [146]. Overall sensitivity was
lower when all lesions were considered (58%) [146].
Disease
detection was PSA dependent, reaching 94% for patients with
a PSA level greater than or equal to 2, and a rate of 61.5%
in patients with a PSA between 0.2-0.5 ng/mL [100].
Detection was also higher (92% vs 78%) in patients that had
received androgen deprivation therapy within the 6 months
before imaging (possibly related to more advanced disease in
these patients) [100].
18F-PSMA
has been shown to have similar diagnostic accuracy to 68Ga-PSMA
for cancer localization in the prostate in newly diagnosed
intermediate or high risk prostate cancer patients [124].
However, 18F-PSMA-1007 has been shown to have a higher rate of non-specific focal bone marrow uptake compared to 68Ga-PSMA-11 and 18F-DCFPyL which requires MRI to further evaluate if there are no findings on the non-diagnostic CT exam [129].
18F-DCFBC:
18F-DCFBC is a PMSA targeted radiotracer [49]. Imaging 2.5 to 3 hours following tracer injection results in improved tumor uptake and decreased background activity [49]. The agent has been shown to detect more lesions than conventional imaging modalities, although it has limited detection for densely sclerotic bone mets and normal liver uptake can mask liver lesions [49].
18F-DCFPyL:
18F-DCFPyL is another PMSA
targeting imaging agent [118,139].
The dose for the exam is 8 mCi and imaging is
started 2 hours following tracer injection [135].
Primary tumor: For the detection of the
primary tumor in the prostate for patients with high risk
prostate cancer, 18F-DCFPyL PET/CT had a sensitivity
of 90.9% and a tumor detection rate of 80% (similar to
mpMRI) [140].
Staging/metastatic disease: One study in a
small number of patients suggested the agent had nearly
identical sensitivity for the detection of metastatic bone
lesions to that of Na18F
[118]. The agent was able to detect marrow and lytic bone
lesions that were not seen on Na18F imaging, but some sclerotic lesions
seen on Na18F
were not identified with the agent [118].
Biochemical recurrence: In one study for the
evaluation of biochemical recurrence, 18F-DCFPyL PET had
an 85% overall positivity rate which increased with higher PSA
levels- 50% for PSA < 0.5 ng/mL; 69% for PSA from 0.5 to
< 1.0; 100% for PSA from 1.0 to < 2.0; 91% for PSA from
2.0 to < 5.0; and 96% for PSA greater than or equal to 5.0
[125]. 18F-DCFPyL PET identified lesions
in 36% of patients who had no findings on conventional
imaging which led to a change in management in 24% of
patients [125]. The agent also localized bone
lesions in 25% of patients with negative results on bone scan [125].
In another study of patients with biochemical
recurrence the detection rates were 48% for PSA 0.21-0.49 ng/mL,
50% for PSA 0.5-0.99, 89% for PSA 1.0-1.99, and 94% for PSA over
2.0 [135]. Extra pelvic disease was found in 30% of patients,
almost exclusively when the PSA was greater than 1 ng/mL [135].
For patients that underwent radical prostectomy, the PSA
doubling time and PSA velocity correlated with the exam results
(but this was not the case for post-radiation patients) [135].
In a prospective study of patients with
biochemical recurrence comparing 18F-DCFPyL PET-CT
to MRI, the agent had an overall sensitivity of 69% (MRI
69%), specificity of 91% (MRI 74%), and PPV of 86% (MRI 69%)
[139]. When 18F-DCFPyL exam
results were combined with MRI findings, the overall PPV
improved to 88% [139]. 18F-DCFPyL
PET-CT depicted more pelvic lymph nodes than did MRI and MRI
depicted more lesions in the prostate bed [139].
Monitoring response to therapy: In patients with metastatic
prostate cancer 18F-DCFPyL uptake measurements have
been shown to be highly repeatable [141]. A change in the
tumor-to-blood ration (TBR) exceeding 32% may indicate a
change in tumoral uptake that exceeds the physiologic
variability [141].
18F-fluoride in prostate cancer:
See also discussion in General
PET Oncologic Imaging
Presently, nuclear medicine bone scan is used to assess for the
presence of bone metastases in patients with high risk prostate
cancer [10]. Bone scanning is indicated for initial staging in
patients with the following criteria: Gleason score 8 or higher;
T1 tumor plus PSA level greater than 20; T2 tumor with a PSA level
greater than 10; T3 or T4 tumor; or symptomatic bone pain [39].
For initial staging, in the detection of osseous metastases, bone
scan has reported sensitivities of 46-89% and specificity of
32-57% [93]. 18F-fluoride has many advantages over
convention bone scan including superior pharmacokinetics, higher
bone uptake, faster blood clearance, lower radiation dose, and
superior image quality [40].
18F-fluoride has been shown to be more sensitive
and accurate than bone scan for the detection and characterization
of bone metastases in cancer patients (detecting more lesions than
bone scan in up to 69% of prostate cancer patients [54])
[10,39,40,61]. In one prospective study of prostate cancer
patients, 18F-fluoride PET had a sensitivity of 100%
for the detection of bone metastases, compared to 70% for
conventional bone scan [10]. In another study, the sensitivity for
the detection of bone metastases was 81%, with a specificity of
93% [16]. Another prospective study found a sensitivity and
specificity of 51% and 82%, respectively for bone scan, 85% and
91% for 11C-choline PET, and 93% and 54% for 18F-fluoride
PET [39]. In the NOPR study, 35% of NaF PET scans demonstrated
more evidence of bone metastases than did bone scan [40]. A
meta-analysis demonstrated a pooled sensitivity of 96% and
specificity of 98%, compared to 57% and 98%, respectively for
planar bone scintigraphy [73]. Interobserver agreement for the
detection of bone metastases in patients with prostate cancer is
also very high [122].
Unfortunately, 18F-fluoride is not tumor specific and
tracer uptake can also be seen in benign bone lesions or
degenerative change [10]. The specificity of 18F-fluoride
PET imaging can be greatly improved through the use of PET/CT
[10]. Fused CT images allow more accurate characterization of
areas of benign tracer uptake [10]. Also- sclerotic lesions noted
on the CT exam which do not demonstrate increased 18F-fluoride
uptake likely reflect benign bone lesions [10]. Overall, PET/CT
imaging improves diagnostic confidence with fewer equivocal exam
interpretations [10].
Initial results from the NOPR study, suggest that NaF PET imaging
in the evaluation of prostate cancer patients (initial staging,
suspected first osseous metastasis, and suspected progression of
osseous metastases) has a high impact on patient management
(changed management in about 40% of cases [122]) and allowed
referring physicians to avoid additional diagnostic tests in about
three quarters of patients [40]. However, the change in management
has not been shown to improve patient outcomes [122].
The baseline number of bone metastases and changes in SUV on followup Na18F PET/CT correlate with overall survival [54].
Other investigational agents:
anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic
acid (anti-3- 18F- FACBC) is a synthetic amino acid
analog taken up by prostate cancer cells, but not further
metabolized [27]. The normal biodistribution of the agent includes
relatively intense uptake in the liver and pancreas and little
renal excretion or brain uptake compared to FDG [27]. The agent
has demonstrated 89% sensitivity and 67% specificity for the
detection of local recurrence in the prostate bed, and 100%
sensitivity for the detection of extraprostatic disease [27].
18F- 16β-fluoro-5-dihydrotestosterone
(FDHT) is an analog of the primary ligand of the androgen
receptor [38].
68Ga-PSMA ligand targets the
membrane bound PSMA receptor which is over-expressed on prostate
cancer cells compared to benign prostatic tissue [45]. The agent
has been shown to be very sensitive for detection of biochemical
recurrent prostate cancer- even in the setting of low PSA levels
(<0.5 ng/mL) [45]. In one study (which unfortunately did not
use biopsy for confirmation in all patients) the agent was able
to detect recurrence in 67% of patients with a PSA < 1.0
ng/nL (compared to reported detection rates of 19-36% for
choline PET tracers at a similar PSA level) [45]. As with
choline tracers, the detection rate is higher in patients with
higher PSA levels and in patients with a tumor with a Gleason
score of greater than or equal to 8 [45].
REFERENCES:
(1) J Nucl Med 2002; Nunez R, et al. Combined 18F-FDG and 11C-methionine PET scans in patients with newly progressive metastatic prostate cancer. 43: 46-55
(2) J Nucl Med 2002; Oyama N, et al. 11C-acetate PET imaging of prostate cancer. 43: 181-186
(3) J Nucl Med 2003; Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. 44: 331-335
(4) J Nucl Med 2003; Oyama N, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. 44: 549-555
(5) J Nucl Med 2003; Dimitrakopoulou-Strauss A, Strauss L. PET imaging of prostate cancer with 11C-acetate. 44: 556-558
(6) Radiol Clin N Am 2004; Kumar R, et al. PET in the management of urologic malignancies. 42: 1141-1153
(7) Radiology 2005; Schmid DT, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. 235: 623-628
(8) J Nucl Med 2005; Farsad M, et al. Detection and localization of prostate cancer: correlation of 11C-choline PET/CT with histopathologic step-section analysis. 46: 1642-1649
(9) J Nucl Med 2006; Kwee SA, et al. Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. 47: 262-269
(10) J Nucl Med 2006; Even-Sapir E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. 47: 287-297
(11) J Nucl Med 2006; Reske SN, et al. Imaging prostate cancer with 11C-choline PET/CT. 47: 1249-1254
(12) J Nucl Med 2007; Ponde DE, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging- in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. 48: 420-428
(13) Radiology 2007; Testa C, et al. Prostate cancer: sextant localization with MR imaging, MR spectroscopy, and 11C-choline PET/CT. 244: 797-806
(14) Radiology 2007; Margolis DJA, et al. Molecular imaging techniques in body imaging. 245: 333-356
(15) J Nucl Med 2008; Vavere AL, et al. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. 49: 327-334
(16) J Nucl Med 2008; Apolo AB, et al. Novel tracers and their development for the imaging of metastatic prostate cancer. 49: 2031-2041
(17) AJR 2009; Kelloff GJ, et al. Challenges in clinical prostate cancer: role of imaging. 192: 1455-1470
(18) AJR 2009; Turkbey B, et al. Imaging localized prostate cancer: current approaches and new developments. 192: 1471-1480
(19) J Nucl Med 2009; Zaheer A, et al. New agents and techniques for imaging prostate cancer. 50: 1387-1390
(20) J Nucl Med 2009; Castellucci P, et al. Influence of trigger PSA and PSA kinetics on 11C-choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. 50: 1394-1400
(21) J Nucl Med 2009; Vilanova JC, et al. Peripheral zone prostate cancer in patients with elevated PSA levels and low free-to-total PSA ratio: detection with MR imaging and MR spectroscopy. 253: 135-143
(22) J Nucl Med 2009; Piert M, et al. Detection of aggressive primary prostate cancer with 11C-choline PET/CT using moltimodality fusion techniques. 50: 1585-1593
(23) Radiology 2010; Beheshti M, et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 120 patients. 254: 925-933
(24) AJR 2010; De Visschere PJ, et al. Role of MRI in follow-up after focal therapy for prostate carcinoma. 194: 1427-1433
(25) J Nucl Med 2011; Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. 52: 81-89
(26) Radiographics 2011; Pano B, et al. Pathways of lymphatic
spread in male urogenital pelvic malignancies. 31: 135-160
(27) Radiology 2011; Schuster DM, et al. Detection of recurrent
prostate carcinoma with anti-1-amino-3- 18F-fluorocyclobutane-1-carboxylic
acid PET/CT and 111In-capromab pendetide SPECT/CT. 259: 852-861
(28) AJR 2011; Murphy RC, et al. The utility of 11C-choline
PET/CT for imaging prostate cancer: a pictoral guide. 196:
1390-1398
(29) J Nucl Med 2012; Mena E, et al. 11C-acetate
PET/CT in localized prostate cancer: a study with MRI and
histopathologic correlation. 53: 538-545
(30) J Nucl Med 2012; Park H, et al. Introducing parametric
fusion PET/MRI of primary prostate cancer. 53: 546-551
(31) AJR 2012; Jadvar H. Molecular imaging of prostate cancer:
PET radiotracers. 199: 278-291
(32) J Nucl Med 2013; 11C-acetate PET/CT before
radical prostatectomy: nodal staging and treatment failure
prediction. 54: 699-706
(33) J Nucl Med 2013; Beheshti M, et al. Impact of 18F-choline
PET/CT
in
prostate cancer patients with biochemical recurrence: influence of
androgen deprivation therapy and correlation with PSA kinetics.
54: 833-840
(34) J Nucl Med 2013; Jadvar H. Molecular imaging of prostate
cancer with PET. 54: 1685-1688
(35) AJR 2013; Murphy G, et al. The expanding role of MRI in
prostate cancer. 201: 1229-1238
(36) J Nucl Med 2014; Kitajima K, et al. Detection of recurrent
prostate cancer after radical prostatectomy: comparison of 11C-choline
PET/CT with pelvic multiparametric MR imaging with endorectal
coil. 55: 223-232
(37) J Nucl Med 29014; Giovacchini G, et al. 11C-choline
PET/CT predicts prostate cancer-specific survival in patients with
biochemical failure during androgen-deprivation therapy. 55:
233-241
(38) Radiology 2014; Vargas HA, et al. Bone metastases in
castration-resistant prostate cancer: associations between
morphologic CT patterns, glycolytic activity, and androgen
receptor expression on PET and overall survival. 271: 220-229
(39) J Nucl Med 2014; Segall GM. PET/CT
with sodium 18F-fluoride
for management of patients with prostate cancer. 55: 531-533
(40) J Nucl Med 2014; Hillner BE, et al.
Impact of 18F-fluoride PET in patients with
known prostate cancer: initial results from the National Oncologic
PET registry. 55: 574-581
(42) J Nucl Med 2015; Oyen WJG, De Jong IJ. Molecular imaging of prostate cancer: tapping into the opportunities. 56: 169-170
(43) J Nucl Med 2015; Cimitan M, et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1000 patients. 56: 209-215
(44) J Nucl Med 2015; Verwer EE, et al. Quantification of 18F-fluorocholine kinetics in patients with prostate cancer. 56: 365-371
(45) J Nucl Med 2015; Eiber M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. 56: 668-674
(46) J Nucl Med 2015; Jadvar H. PSMA PET in prostate cancer. 56: 1131-1132
(47) J Nucl Med 2015; Morigi JJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. 56: 1185-1190
(48) J Nucl Med 2015; Incerti E, et al. Radiation treatment of lymph node recurrence from prostate cancer: is 11C-choline PET/CT predictive of survival outcomes? 56: 1836-1842
(49) J Nucl Med 2016; Rowe SP, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone naive and castrate-resistant metastatic prostate cancer. 57: 46-53
(50) Radiographics 2016; Wibmer AG, et al. Molecular imaging of prostate cancer. 36: 142-161
(51) Radiographics 2016; Welle CL, et al. 11C-choline PET/CTin recurrent prostate cancer and nonprostatic neoplastic processes. 36: 279-292
(52) J Nucl Med 2016; Rahbar K, et al. Correlation of intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. 57: 563-567
(53) J Nucl Med 2016; Oprea-Lager DE, et al. Repeatability of quantitative 18F-fluoromethylcholine PET/CT studies in prostate cancer. 57: 721-727
(54) J Nucl Med 2016; Apolo AB, et al. Prospective study evaluating Na18F PET/CT in predicting clinical outcomes and survival in advanced prostate cancer. 57: 886-892
(55) J Nucl Med 2016; Lee J, et al. Prediction of PSA progression in castration-resistant prostate cancer based on treatment-associated change in tumor burden quantified by 18F-fluorocholine PET/CT. 57: 1058-1064
(56) J Nucl Med 2016; FDA approves 18F-Fluciclovine and 68Ga-DOTATATE products. 57: 9N-10N
(57) J Nucl Med 2007; Schuster DM, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. 48: 56-63
(58) J Nucl Med 2016; Kratochwil C, et al. PMSA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PMSA-617. 57: 1170-1176
(59) J Nucl Med 2016; McConathy J. 18F-Fluciclovine (FACBC) and its potential use for breast cancer imaging. 57: 1329-1330
(60) J Nucl Med 2016; Chen FK, et al. Utility of ultrasound in the diagnosis, treatment, and follow-up of prostate cancer: state of the art. 57: 13S-18S
(61) J Nucl Med 2016; Iagaru AH, et al. Bone-targeted imaging and radionuclide therapy in prostate cancer. 57: 19S-24S
(62) J Nucl Med 2016; Jadvar H. PET of glucose metabolism and cellular proliferation. 57: 25S-29S
(63) J Nucl Med 2016; Nitsch S, et al. Evaluation of prostate cancer with 11C- and 18F-choline PET/CT: diagnosis and initial staging. 57: 38S-42S
(64) J Nucl Med 2016; Mapelli P, et al. 11C- or 18F-choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. 57: 43S-48S
(65) J Nucl Med 2016; Ceci F, et al. Evaluation of prostate cancer with 11C-choline PET/CT for treatment planning, response assessment, and prognosis. 57: 49S-54S
(66) J Nucl Med 2016; Beheshti M, et al. Evaluation of prostate cancer bone metastases with 18F-NaF and 18F-choline PET/CT. 57: 55S-60S
(67) J Nucl Med 2016; Fendler WP, et al. 68Ga-PMSA PET/CT detects the localization and extent of primary prostate cancer. 57: 1720-1725
(68) AJR 2016; Haroon A, et al. 18F-FECH PET/CT to assess clinically significant disease in prostate cancer: correlation with maximum and total cancer core length obtained via MRI-guided template mapping biopsies. 207: 1297-1306
(69) Radiology 2017; Choi JY, et al. 18F fluorocholine dynamic time-of-flight PET/MR imaging in patients with newly diagnosed intermediate- to high-risk prostate cancer. initial clinical-pathologic comparisons. 282: 429-436
(70) J Nucl Med 2017; Dietlein F, et al. PSA-stratified performance of 18F and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. 58: 947-952
(71) J Nucl Med 2017; Ulaner GA, et al. Prospective clinical trial of 18F-fluciclovine PET/CT for determining the response to neoadjuvant therapy in invasive ductal and invasive lobular breast cancers. 58: 1037-1042
(72) J Nucl Med 2017; Einspieler I, et al. Detection efficacy of hybrid 68Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by Phoenix criteria. 58: 1081-1087
(73) Radiographics 2017; Wallitt KL, et al. Clinical PET imaging in prostate cancer. 37: 1512-1536
(74) J Nucl Med 2017; Schwarzenboeck SM, et al. PMSA ligands for PET imaging of prostate cancer. 58: 1545-1552
(75) J Nucl Med 2017; Fedler WP, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. 58: 1617-1623
(76) J Nucl Med 2017; Eiber M, et al. Prostate-specific membrane antigen ligands for imaging and therapy. 58: 67S-76S
(77) Radiology 2017; Thoeny HC, et al. Functional and targeted lymph node imaging in prostate cancer: current status and future challenges. 285: 728-743
(78) J Nucl Med 2017; Osman MM, et al. Detection of synchronous primary malignancies with 68Ga-labeled prostate-specific membrane antigen PET/CT in patients with prostate cancer: frequency in 764 patients. 58: 1938-1942
(79) J Nucl Med 2017; Koerber SA, et al. 68Ga-PMSA-11 PET/CT in newly diagnosed carcinoma of the prostate: correlation with intraprostatic PMSA tracer uptake with several clinical parameters. 58: 1943-1948
(80) J Nucl Med 2017; Vinsenia M, et al. 68Ga-PSMA PET/CT and volumetric morphology of PET-positive lymph nodes stratified by tumor differentiation of prostate cancer. 58: 1949-1955
(81) J Nucl Med 2017; Hope TA, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. 58: 1956-1961
(82) J Nucl Med 2017; Emmett L, et al. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. 58: 1972-1976
(83) Radiographics 2018; Hofman MS, et al. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. 38: 200-217
(84) J Nucl Med 2018; Roach PJ, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. 59: 82-88
(85) J Nucl Med 2018; Donin NM, Reiter RE. Why targeting PMSA is a game changer in the management of prostate cancer. 59: 177-182
(86) J Nucl Med 2018; Calais J, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. 59: 230-237
(87) J Nucl Med 2018; Woythal N, et al. Immunohistochemical validation of PMSA expression measured by 68Ga-PSMA PET/CT in primary prostate cancer. 59: 238-243
(88) J Nucl Med 2018; Calais J, et al. Impact of 68Ga-PSMA PET/CT on the management of prostate cancer patients with biochemical recurrence. 59: 434-441
(89) J Nucl Med 2018; Parent EE, Schuster DM. Update on 18F-fluciclovine PET for prostate cancer imaging. 59: 733-739
(90) J Nucl Med 2018; Elschot M, et al. Combined 18F-fluciclovine PET/MRI show potential for detection and characterization of high-risk prostate cancer. 59: 762-768
(91) J Nucl Med 2018; Calais J, et al. Comparison of 68Ga-PSMA-11 and 18F-fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. 59: 789-794
(92) J Nucl Med 2018; Kratochwil C, et al. Targeted alpha-therapy of metastatic castrate resistant prostate cancer with 225Ac-PMSA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. 59: 795-802
(93) J Nucl Med 2018; Calais J, et al. The utility of PET/CT in the planning of external radiation therapy for prostate cancer. 59: 557-567
(94) AJR 2018; Taneja S, et al. Effect of combined 68Ga-PSMA-HBED-CC uptake pattern and multiparametric MRI derived with simultaneous PET/MRI in the diagnosis of primary prostate cancer: initial experience. 210: 1338-1345
(95) J Nucl Med 2018; Ahmadzadehfar H, Essler M. Predictive factors of response and overall survival in patients with castration-resistant metastatic prostate cancer undergoing 177Lu-PMSA therapy. 59: 1033-1034
(96) Radiology 2018; Park SY, et al. Gallium 68-PMSA-11 PET/MR imaging in patients with intermediate- or high risk prostate cancer. 288: 495-505
(97) Radiology 2018; Hicks RM, et al. Diagnostic accuracy of 68Ga-PSMA-11 PET/MRI compared with multiparametric MRI in the detection of prostate cancer. 289: 730-737
(98) J Nucl Med 2019; Schmidt-Hegemann NS, et al. Outcome after PMSA PET/CT-based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a 2-institution retrospective analysis. 60: 227-233
(99) J Nucl Med 2019; Koerber SA, et al. 68Ga-PSMA-11 PET/CT in primary and recurrent prostate carcinoma: implications for radiotherapeutic management in 121 patients. 60: 234-240
(100) J Nucl Med 2019; Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. 60: 362-368
(101) J Nucl Med 2014; Schuster DM, et al. Anti-1-Amino-3-18F-Fluorocyclobutane-1-Carboxylic Acid: Physiologic Uptake Patterns, Incidental Findings, and Variants That May Simulate Disease. 55: 1986-1992
(102) Radiographics 2019; Gusman M, et al. Review of 18F-fluciclovine PET for detection of recurrent prostate cancer. 39: 822-841
(103) J Nucl Med 2019; Hope TA, et al. Metaanalysis of 68Ga-PSMA-11 PET accuracy for the detections of prostate cancer validated by histopathology. 60: 786-793
(104) J Nucl Med 2019; Emmett L, et al. Rapid modulation of PMSA expression by androgen depreviation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. 60: 950-954
(105) J Nucl Med 2019; Barber TW, et al. Clinical outcomes of 177Lu-PMSA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. 60: 955-962
(106) J Nucl Med 2019; Schmidt-Hegemann NS, et al. Impact of 68Ga-PSMA PE/CT on the radiotherapeutic approach to prostate cancer in comparison to CT: a retrospective analysis. 60: 963-970
(107) J Nucl Med 2019; Jilg CA, et al. Detection rate of 18F-choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: dependence on the size of the tumor deposits in lymph nodes. 60: 971-977
(108) AJR 2019; Subramaniam RM. Prostate cancer theranosis in clinical practice and in clinical trials- 68Ga-prostate-specific member antigen (PSMA)-11 PET/CT and 177Lu-PMSA-617 therapy. 213: 241-42
(109) AJR 2019; Yadav MP, et al. Radioligand therapy with 177Lu-PMSA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. 213: 275-285
(110) AJR 2019; Osmany S, et al. Gallium-68-labeled prostate-specific membrane antigen-11 PET/CT of prostate and nonprostate cancers. 213: 286-299
(111) J Nucl Med 2019; Burger IA, et al. 68Ga-PSMA PET/MR detects local recurrence occult on mpMRI in prostate cancer patients after HIFU. 60: 1118-1123
(112) AJR 2019; Savir-Baruch B, et al. Fluorine-18-labeled fluciclovine PET/CT in clinical practice: factors affecting the rate of detection of recurrent prostate cancer. 213: 851-858
(113) J Nucl Med 2019; Ghafoor S, et al. Multimodality imaging of prostate cancer. 60: 1350-1358
(114) J Nucl Med 2019; Yilmaz B, et al. Effect of external cooling on 177Lu-PMSA uptake by the parotid glands. 60: 1388-1393
(115) J Nucl Med 2019; Ekmekcioglu O, et al. Bridging the imaging gap: PMSA PET/CT has a high impact on treatment planning in prostate cancer patients with biochemical recurrence- a narrative review of the literature. 60: 1394-1398
(116) J Nucl Med 2020; Koerber SA, et al. Lymph node involvement in treatment-naive prostate cancer patients: correlation of PMSA PET/CT imaging and Roach formula in 280 men in radiotherapeutic management. 61: 46-50
(117) J Nucl Med 2020; Sathekge M, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PMSA-617 radioligand therapy. 61: 62-69
(118) J Nucl Med 2020; Rowe SP, et al. Prospective comparison of PET imaging with PMSA targeted 18F-DCFPyL versus Na18F for bone lesion detection in patients with metastatic prostate cancer. 61: 183-188
(119) J Nucl Med 2020; Rauscher I, et al. Can the injected dose be reduced in 68Ga-PSMA-11 PET/CT while maintaining high image quality for lesion detection? 61: 189-193
(120) J Nucl Med 2020; Krabzbuhler B, et al. Detection rate and localization of prostate cancer recurrence using 68Ga-PSMA-11 PET/MRI in patients with low PSA values
(121) AJR 2020; Robertson MS, et al. Extraprostatic uptake of 18F-fluciclovine: differentiation of nonprostatic neoplasms from metastatic prostate cancer. 214: 641-648
(122) J Nucl Med 2020; Zacho HD, et al. Observer agreement and accuracy of 18F-sodium flouride PET/CT in the diagnosis of bone metastases in prostate cancer. 61: 344-349
(123) J Nucl Med 2020; Pomykala KL, et al. 68Ga-PMSA-11 PET/CT for bone metastases detection in prostate cancer patients: potential impact on bone scan guidelines. 61: 405-411
(124) J Nucl Med 2020; Kuten J, et al. Head-to-head comparison of 68Ga-PMSA-11 with 18F-PMSA-1007 in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. 61: 527-532
(125) J Nucl Med 2020; Song H, et al. Prospective evaluation of 18F-DCFPyL PET/CT in biochemically recurrent prostate cancer in an academic center: a focus on disease localization and changes in management. 61: 546-551
(126) J Nucl Med 2020; Jadvar H, et al. Appropriate use criteria for imaging evaluation of biochemical recurrence of prostate cancer after definitive primary treatment. 61: 552-562
(127) J Nucl Med 2016; Baum RP, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. 1006-1013
(128) J Nucl Med 2020; Rathke H, et al. Response prediction of 177Lu-PMSA-617 radioligand therapy using prostate-specific antigen, chromogranin A, and lactate dehydrogenase. 61: 689-695
(129) J Nucl Med 2020; Dietlein F, et al. Intraindividual comparison of 18F-PMSA-1007 with renally excreted PMSA ligands for PMSA PET imaging in patients with relapsed prostate cancer. 61: 729-734
(130) Radiographics 2020; Tanaka T, et al. Current imaging techniques for and imaging spectrum of prostate cancer recurrence and metastasis: a pictorial review. 40: 709-726
(131) AJR 2020; Bhargava P, et al. Imaging biochemical recurrence after prostatectomy: where are we headed? 214: 1248-1258
(132) J Nucl Med 2020; Cook GJR, Goh V. Molecular imaging of bone metastases and their response to therapy. 61: 799-806
(133) J Nucl Med 2020; Michaud L, et al. 11C-choline PET/CT in recurrent prostate cancer: retrospective analysis in a large U.S. patient series. 61: 827-833
(134) J Nucl Med 2020; Emmett L, et al. 3-year freedom from progression after 68Ga-PMSA PET/CT-triaged management in men with biochemical recurrence after radical prostatectomy: results of a prospective multicenter trial. 61: 866-872
(135) J Nucl Med 2020; Mena E, et al. 18F-DCFPyL PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. 61: 881-889
(136) Radiology 2020; Tan N, et al. PMSA-targeted radiotracers versus 18F fluciclovine for the detection of prostate cancer biochemical recurrence after definitive therapy: a systemic review and meta-analysis. 296: 44-55
(137) Lancet Oncol 2019; Calais J, et al. 18F-fluciclovine PET-CT and 68Ga-PMSA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-center, single-arm, comparative imaging trial. 20: 1286-1294
(138) AJR 2020; Marcus C, et al. Fluorine-18-labeled fluciclovine PET/CT in primary and biochemically recurrent prostate cancer management. 215: 267-276
(139) Radiology 2020; Lindenberg L, et al. Evaluating biochemically recurrent prostate cancer: histologic validation of 18F-DCFPyL PET/CT with comparison to multiparametric MRI. 296: 564-575
(140) AJR 2020; Gaur S, et al. Prospective evaluation of 18F-DCFPyL PET/CT in detection of high-risk localized prostate cancer: comparison with mpMRI. 215: 652-659
(141) J Nucl Med 2020; Jansen BHE, et al. Repeatability of quantitative 18F-DCFPyL PET/CT measurements in metastatic prostate cancer. 61: 1320-1325
(142) Radiographcis 2020; Barbosa FG, et al. Prostate-specific membrane antigen PET: therapy response assessment in metastatic prostate cancer. 40: 1412-1430
(143) Radiographics 2020; Barwick TD, Castellucci P. Invited commentary: prostate-specific membrane antigen PET response assessment- has time come? 40: 1431-1433
(144) AJR 2020; Wang Y, et al. Utility of 18F-fluciclovine PET/CT for detecting prostate cancer recurrence in patients with low (< 1 ng/mL) or very low (< 0.3 ng/mL) prostate-specific antigen levels. 215: 997-1001
(145) J Nucl Med 2020; Beheshti M, et al. Multiphasic 68Ga-PMSA PET/CT in the detection of early recurrence in prostate cancer patients with a PSA level of less than 1 ng/mL: a prospective study of 135 patients. 61: 1484-1490
(146) J Nucl Med 2021; Sprute K, et al. Diagnostic accuracy of 18F-PMSA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. 62: 208-213
(147) J Nucl Med 2021; 68Ga-PMSA PET/CT for primary lymph node and distant metastasis in NM staging of high-risk prostate cancer. 62: 214-220
(148) AJR 2021; Satapathy S, et al. Diagnsotic accuracy of 68Ga-PMSA PET/CT for initial detection in patients with suspected prostate cancer: a systematic review and meta-analysis. 216: 599-607
(149) AJR 2021; Savir-Baruch B, et al. Role of 18F-fluciclovine and prostate-specific membrane antigen PET/CT in guiding management of oligometastatic prostate cancer: AJR expert panel narrative review. 216: 851-859