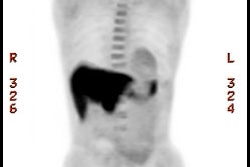
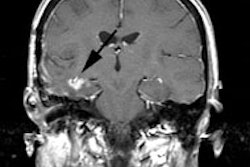
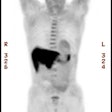
Islet Cell / Pancreatic Neuroendocrine Tumors:
(See also Octreotide Imaging of Islet Cell Tumors)
Clinical:
Islet cell tumors were felt to arise from APUD cells and are hormonally active in 85% of cases. However, new evidence suggests that pancreatic neuroendocrine tumors (NETs) originate from pluripotential stem cells in ductal epithelium [13]. Pancreatic NETs account for 1-5% of all pancreatic tumors [13]. The tumors can be functioning (15-52% of cases) or non-functioning [13]. Data now suggest that non-functioning tumors are the most common- accounting for 60-80% of pancreatic NETs [16]. The tumors are also classified based on histopatholgic findings into two broad categories as either well-differentiated pancNET (typically these tumors have minimal to moderate atypia, lack necrosis, and express intense synaptophysin or chromogranin A positivity) or poorly differentiated pancNET (typically consisting of atypical cells, substantial necrosis, and faint positivity for neuroendocrine tumor markers) [20]. With the use of the mitotic index (number of mitoses per 10 high power fields) and the Ki-67 index the tumors are classified into 3 grades [20]. The latest classification system recognizes two categories of high Ki-67/mitotic index tumors- well-differentiated high grade tumors and poorly differentiated carcinomas (PancNEC) [20]. PancNECs are aggressive, large, heterogeneous (cystic change, necrosis, and calcification are common), poorly differentiated neoplasms with ill-defined margins composed of atypical cells characterized by TP53 and Rb1 mutations, and less commonly by KRAS and SMAD4 mutations, which differentiates them from PNETs [20].WHO classification [20]:
| Classification |
Mitotic index |
Ki-67 |
| Well-differentiated pancNET, grade 1 |
<2 |
|
| Well-differentiated pancNET, grade 2 |
20 |
3-20 |
| Well-differentiated pancNET, grade 3 |
>20 |
>20 |
| Poorly differentiated panc-neuroendocrine
carcinoma |
>20 |
>20 |
There is a higher risk aggressive malignant potential if the lesion is non-functional (50-90% are malignant) [16]. The risk of malignancy is also related to tumor size- especially if larger than 5cm [13]. Pancreatic NETs, even malignant lesions, generally show a more indolent behavior and have a better prognosis than pancreatic adenocarcinoma [16]. The five year survival rate for pancreatic neuroendocrine tumors is 81%, which is significantly higher than that of pancreatic adenocarcinoma [15]. The tumor grade, AJCC stage, and presence of metastatic disease are the most important prognostic factors [20].
Complete surgical resection with regional lymph node dissection is the treatment of choice for well-differentiated grade 1 and grade tumors (and should be considered for all patients with early stage disease) [20]. Grade 3 PNETs with a lower Ki-67 index (21-55%) have a better prognosis with the use of an aggressive surgical approach [20]. The mTOR inhibitor everolimus and tyrosine kinase inhibitor sunitini have shown promise in progression free survival in patients with grade 3 well-differentiated PNETs [20]. Peptide receptor radiotherapy is another option for treatment of grade 3 PNETs [20].
Most cases of pancreatic NETs are sporadic (75%), but they can also be associated with genetic syndromes (10% of cases [20]) such as MEN I, von-Hippel Lindau syndrome, neurofibromatosis type I, tuberous sclerosis, and glucagon cell adenomatosis [13,16,20].
MEN I (Wermer's syndrome) is charaterized by pancreatic islet cell tumor, pituitary adenoma, and parathyroid adenoma or hyperplasia. MEN I is an autosomal dominant disorder due to a genetic defect on chromosome 11 in the MEN1 gene. It is the most common genetic syndrome associated with PNETs accounting for approximately 10% of all tumors [20]. Patients with this syndrome are more likely to have multiple tumors, which behave in a more aggressive (malignant) manner. Gastrinomas (multifocal gastrinomas) are the most common islet cell tumor found in patients with MEN I (approximately 50% [20%-60%]of patients) [20]. Insulinomas are the next most frequent islet cell tumor found in these patients [2], however, other authors suggest non-function PNETs are more common [20]. Cystic tumors consitute up to 15% of all PNETs in patients with MEN1 (typically large, likely benign, and mostly non-functioning [20]. The most common type of pituitary tumor is a prolactin-producing adenoma, typically a macroadenoma [17].
In von Hippel-Lindau- islet cell tumors may occur in 5-17% of VHL patients (typically presenting at an earlier age than sporadic PNETs)- more commonly in VHL patients with pheochromocytomas [11,20]. Approximately 50% of PNETs in VHL patients are small (< 2 cm) and multiple PNETs are often seen (mostly nonfunctioning) [20]. In VHL patients, the frequency of malignancy and metastases is low (less than 10-15%) and the tumors are typically non-functional [11,20].
1- Insulinoma: This is the most common functioning tumor
[10], it arises from the beta cells. Whipple's triad refers to,
low serum glucose levels (serum glucose< 40-50 ng/dL), symptoms
of hypoglycemia such as dizziness or weakness associated with
fasting or exercise, and relief of symptoms with glucose [10].
Insulinomas are almost invariably located within the pancreas
(99%). Patients may also complain of sweating, palpatations,
and tremor in response to hypoglycemic catecholamine release. Most
insulinomas behave in a benign fashion (90%) and they are
generally solitary (usually less than 2cm in size). About 10% are
multiple [7,10]. About 5-10% are associated with MEN I [10] and in
these situations, there may be multiple lesions. Treatment is
surgical excision.
The lesion is very vascular and will enhance with IV contrast. Transabdominal ultrasound has a sensitivity of 19-60% for the detection of insulinomas (mean 46%) [1]. CT has a sensitivity of 28-79% (mean 38%) [1]. The addition of pancreatic perfusion color images to biphasic contrast CT has been suggested to improve the detection of insulinomas [18]. Calcification can be found in up to 20% of lesions on CT [4].
2- Gastrinoma: The second most common functioning islet
cell tumor [7,10]. Gastrinomas are commonly multiple and are often
extrapancreatic in location (the gastrinoma triangle is bordered
by the junction of the 2nd and 3rd parts of the duodenum, the
pancreatic head, and the confluence of the cystic and common bile
ducts) [10,20]. Excess amounts of gastrin (fasting levels greater
than 1,000 ng/L) lead to stimulation and hyperplasia of the
parietal cells in the gastric fundus which results in a 10 to 20
fold increase in gastric acid production. Patients present with
intractable gastric ulcer disease. This is referred to as
Zollinger-Ellison syndrome. Patients may also experience diarrhea
because of the gatric hypersecretion. Most of the ulcers which
occur in association with gastrinomas are in the stomach and
duodenum, but 10-30% will be in atypical sites such as the
duodenum or proximal jejunum, stomach, or splenic hilum. There is
a solitary ulcer in 90% of cases. Thickened folds are seen within
the stomach, duodenum, and proximal jejunum. Most gastrinomas
behave in a malignant manner (60-70%).
Up to 90% of tumors and malignant [20] and metastases are present
in about 30-50% of cases at the time of diagnosis. Hepatic
metastases are associated with a poor prognosis, while patients
with isolated lymph node metastases often have long-term survival
[7]. Multiple tumors are found in about 10% of cases and this is
associated with MEN I syndrome [9]. Treatment consists of total
gastrectomy if the tumor cannot be located. 68Ga-DOTATATE
PET imaging shows intense tracer uptake in gastrinomas [20].
3- Glucagonoma: This tumor arises from the alpha cells. Men and women are affected equally [10]. Patients present with hyperglycemia (diabetes), stomatitis, weight loss, anemia, and migratory necrolytic erythema (a characteristic necrotizing skin rash found in more than 70% of cases [10]). The rash characteristically involves the lower extremities and perineum/genitals [7,10]. Deep venous thrombosis and pulmonary emboli may also occur [10]. The lesion can be associated with the 4D syndrome: dermatosis, diarrhea, depression, and DVT [10]. Most glucagonomas behave in a malignant manner [10]. Five year survival is about 50% [7].
4- VIPoma: (Vasoactive intestinal peptide) Patients present with Verner-Morrison syndrome- watery diarrhea, hypokalemia and achlorhydria (WDHA). About 50-80% are malignant and liver metastases are common at the time of diagnosis [10]. Most VIPomas are located in the pancreas- with 75% being in the tail [10].
5- Somatostatinoma: Patients present with the triad of diabetes, steatorrhea, and gallstones. May rarely produce a mega-gallbladder due to the inhibitory effects on of somatostatin on CCK. Metastatic disease is present in up to 70% of patients at the time of diagnosis. A family history of islet cell tumors is found in 50% of cases.
6- Non-functional: Non-functioning tumors now account for 60-80% of pancreatic neuroendocrine tumors [14]. Non-functioning islet cell tumors (also called non-functioning neuroendocrine tumors) that come to clinical attention are almost always malignant (80-90% are malignant) [12]. They are the third most frequent endocrine pancreatic tumor (after insulinoma and gastrinoma) and account for 30-35% of all pancreatic neuroendocrine tumors [12]. They are relatively slow growing and are usually quite large (over 6cm) by the time of presentation. In many cases, malignancy can only be confirmed by identification of invasion of adjacent organs or the presence of distant metastases [12]. Metastases are found in 10% of patients, but generally patients still have a relatively long survival [6]. Patients with von Hippel-Lindau disease have an increased risk for non-functioning pancreatic neuroendocrine tumors (seen in about 5% of patients) [6]. The margins of the tumor are often somewhat better defined than with adenocarcinoma. Central necrosis is common and calcification is seen in up to 25% of cases. Non-functioning tumors are also frequently hypervascular. Surgery is the only curative therapy for nonfunctioning pancreatic neuroendocrine tumors [12].
X-ray:
Sonography: It is often difficult to localize most hormonally active tumors as they are usually very small and transabdominal ultrasound is less effective for identifying tumors less than 2 cm in size. Transabdominal ultrasound has a sensitivity of 61-63% (sensitivity is 20-30% for gastrinoma, and 20-75% for insulinoma). Endoscopic ultrasound is more useful for the identification of small lesion (sensitivity 77-94%). Most islet cell tumors appear as well circumscribed areas of decreased echogenicity, but with increased vascularity [20]. Intraoperative ultrasound may be useful as the tumor frequently appears as a hypoechoic mass and has a reported sensitivity of 82% (75-100%).Hepatic metastases typically appear hyperechoic [20].Computed Tomography: On CT, hypervascular lesions enhance
following IV contrast, but they are not commonly seen (Sensitivity
57-63%) [3,8]. Also- up to 41.5% of pancreatic neuroendocrine
tumors may not show arterial hyperenhancement [19]. However, dual
phase multidetector CT imaging with the use of thin (2mm) sections
can improve the sensitivity of CT to about 94% [8]. Larger lesions
tend to show heterogenenous enhancement [13]. Calcification can be
found in up to 20% of pancreatic neuroendocrine tumors (as opposed
to only 2% of pancreatic adenocarcinomas [13]. Metastases are
typically hyperenhancing during the aterial phase and remain
mildly hyperattenuating during portal venous and delayed phases
[20].
Magnetic Resonance Imaging: T1 weighted SE sequences with
fat suppression are excellent for depicting islet cell tumors [1].
Non-enahanced spoiled GRE images can also be used to identify the
lesions and require less acquisition time than T1 SE images [1].
Islet cell tumors are typically high signal on T2 weighted images
and fat-suppressed inversion recovery images [2]. A torso array
coil is recommended for better delineation as the lesions are
often small [1]. PNETs larger than 2 cm with ill-defined margins
that show heterogeneous enhacement, hypoenhancement, or low
apparent diffusion coefficients (ADCs) suggest a high grade lesion
[20]. Grades 2 and 3 PNETs can be recognized by an absolute ADC
cutoff value of 1.21 x 10-3 mm2/sec [20]. A
higher tumor-to-background ratio predicts a lower tumor grade,
with cutoff values of 1.03 for grade 1 tumors [20].
Angiography: Virtually all islet cell tumors demonstrate an intense stain in the late arterial and capillary phases. Other pancreatic neoplasms, particularly adenocarcinoma, are typically hypovascular [2].
REFERENCES:
(1) Radiology 2000; Thoeni RF, et al. Detection of small,
functional islet cell tumors in the pancreas: Selection of MR
imaging sequences for optimal sensitivity. 214: 483-490
(2) Radiographics 1997; 17: 453-472
(3) AJR 1995; Van Hoe L, et al. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images.165:1437-9.
(4) AJR 2002; Lesniak RJ, et al. Spectrum of causes of pancreatic calcifications. 178: 79-86
(5) AJR 2002; Sheth S, et al. Helical CT of islet cell tumors of the pancreas: typical and atypical manifestations. 179: 725-730
(6) Radiology 2002; Marcos HB, et al. Neuroendocrine tumors of the pancreas in von Hippel-Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. 225: 751.
(7) AJR 2002; Demos TC, et al. Cystic lesions of the pancreas. 179: 1375-1388
(8) AJR 2003; Gouya H, et al. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. 181: 987-992
(9) Radiographics 2006; Scarsbrook AF, et al. Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach. 26: 433-451
(10) Radiographics 2006; Horton KM, et al. Multi-detector row CT of pancreatic islet cell tumors. 26: 453-464
(11) Radiographics 2008; Leung RS, et al. Imaging features of von Hippel-Lindau disease. 28: 65-79
(12) AJR 2009; Malago R, et al. Contrast-enhanced sonography of
nonfunctioning pancreatic neuroendocrine tumors. 192: 424-430
(13) Radiographics 2011; Low G, et al. Multimodality imaging of
neoplastic and nonneoplastic solid lesions of the pancreas. 31:
993-1015
(14) Radiographics 2012; Sahani DV, et al. State-of-the-art
PET/CT of the pancreas: current role and emerging indications. 32:
1133-1158
(15) AJR 2012; Coakley FV, et al. Pancreatic imaging mimics: Part
I, imaging mimics of pancreatic adenocarcinoma. 199: 301-308
(16) AJR 2013; Gallotti A, et al. Incidetnal neuroendocrine
tumors of the pancreas: MDCT findings and features of malignancy.
200: 355-362
(17) Radiographics 2017; Vijapura C, et al. Genetic syndromes
associated with central nervous system tumors. 37: 258-280
(18) AJR 2017; Zhu L, et al. Insulinoma detection with MDCT: is
there a role for whole-pancreas perfusion? 208: 306-314
(19) Radiology 2017; Jeon SK, et al. Nonhypervascular pancreatic
neuroendocrine tumors: differenital diagnosis from pancreatic
ductal adenocarcinomas at MR imaging- retrospective
cross-sectional study. 284: 77-87