Neuroendocrine Tumors
Also see sections on Pancreatic
NETs and Octreotide
imaging
Clinical:
Gastroenteropancreatic neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from cells of the neuroendocrine system [23,27]. In 2010, the WHO classification was revised and carcinoid and islet cell tumors are now categorized as NETs (carcinoid tumors are NETs that arise from amine precursor uptake decarboxylase tumors or APUDomas)) [27,28]. NET's account for about 1.5% of all gastrointestinal and pancreatic neoplasms [23]. Most cases are sporadic with a median age of 63 years at the time of diagnosis [23]. However, up to 25% of NETs are associated with a genetic syndrome such as MEN 1 (Wermer syndrome- NETs are found in 25-75% of patients with MEN 1 (most commonly nonfunctioning and gastrinomas), neurofibromatosis type 1 (rare incidence of somatostatinomas, but frequently associated with metastatic disease in 30%), or von Hipple Lindau disease (VHL) [23]. Pancreatic NETs develop in 15% (9-17%) of VHL patients with a mean age of onset of 35 years typically in the head and uncinate process, about 53% are multiple, and the lesions typically non-functioning [58].
The classic carcinoid syndrome is seen in about 10-15% of all patients with GI NETs and is characterized by cutaneous flushing, diarrhea, abdominal pain, wheezing, palpatations, and low blood pressure [27]. The syndrome is related to serotonin and kallikrein secretion by the tumors and the syndrome typically occurs in the setting of extensive hepatic metastases [27]. The symptoms are often aggravated by exercise, drinking red wine, or eating certain foods such as cheese and chocolate [27].
NETs tend to be generally slow-growing, but approximately two thirds will have metastases at the time of diagnosis [31]. The incidence of metastases depends on the site of the primary tumor, tumor size, depth of invasion, and tumor differentiation [27]. For example, only 5% of rectal NETs have mets at presentation, compared to 30% of jejunoileal lesions, and 44% of cecal lesions [27]. Midgut tumors less than 2cm are associated with up to 80% of nodal and 50% liver metastases, compared with 20-30% in tumors less than 1 cm [27]. In general, tumors that invade beyond the muscularis propria have a greater incidence of metastases [27]. The tumor grade also affects the likelihood for mets with the development of metastatic disease in 50% of poorly differentiated lesions compared to 20% in well-differentiated tumors [27].
NETs can produce metabolically active hormones and amines [23]. Approximately 15-30% of gastrointestinal NETs are functioning tumors with hormone-related symptoms, and 70-85% are nonfunctioning [28]. 5-HIAA is a serotonin breakdown product that is excreted in the urine [27]. 5-HIAA levels are elevated in 75% of midgut carcioids, but in only 30% of foregut lesions, and in 0% of hindgut NETs [27]. Chomogranin A is the most commonly used neuroendocrine serum marker (elevated in 80-90%) of GI NETs including foregut, midgut, and hindgut carcinoids) and elevated levels are associated with a higher tumor burden [23,27]. Chomogranin A levels are also higher in patients with well-differentiated tumors [23]. Chomogranin A levels can be falsely elvated in patients treated with proton pump inhibitors [23]. Chomogranin A has a reported sensitivity 68% and specificity 86% for the detection of NETs [23].
The World Health Organization has proposed classifying gastroenteropancreatic neuroendocrine tumors (NETs) as well-differentiated tumors, well-differentiated carcinomas, and poorly differentiated carcinomas (grade I, II, or III) based on histology (degree of de-differentiation) and proliferative index (Ki-67 proliferative index and mitotic count) [9,27]. The well-differentiated neoplasms, regardless of their benign or malignant behavior, are classified as neuroendocrine tumors and graded Grade 1 (Ki67 < /=2% and fewer than 2 mitoses per 10 high power fields) or Grade 2 (Ki67 of 3% to 20% or 2-20 mitoses per 10 HPF ) [35,69,76]. The poorly differentiated neoplasms are classified as neuroendocrine carcinomas and graded G3 (Ki67 > 20% or more than 20 mitoses per 10 HPF ) [35,37,62,76]. The loss of differentiation in G3 tumors is generally associated with a loss of SSTR expression, an increase in glycolytic metabolism, and an increase in tumor aggressiveness [76].
Most NETs are well differentiated (G1/G2) are relatively indolent, but between 6-8% are G3 and these lesions behave more aggressively [55,56]. The 5-year survival rate for grade 1 tumors is estimated to be 89%, compared with 70% for grade 2 tumors, and less than 57% for grade 3 lesions [65]. Poorly differentiated NETs respond to cisplatin therapy in more than 50% of cases, however, G3 lesions with a Ki-67 index of less than 55% have been shown to have a lower response rate [62]. Hence, G3 tumors are subdivided into two groups- well differentiated (Ki-67 20-50%) and poorly differentiated (Ki-67 > 50%) [62,64].
Most NETs are well differentiated and with the exception of benign insulinomas, generally demonstrate the presence/overexpression of cell surface receptors for somatostatin- especially subtype 2 (there are five subtypes of somatostatin receptors) [4,6,16,60]. These well differentiated lesions are also slow growing and therefore they have lower metabolic rates [4]. Consequently, their relatively lower glucose utilization results in a lower sensitivity for their detection on FDG imaging [4]. FDG PET imaging is useful only in patients who are suspected of having aggressive neuroendocrine tumors [4]. Most patients with metastatic neuroendocrine tumors that are FDG positive (or somatostatin receptor imaging negative) demonstrate early progressive disease and decreased overall survival despite treatment [56].
Somatostatin analogue treatment with octreotide or lanreotide is given to slow or stop the progression of the disease and to palliate hormonal syndromes, rather than provide tumoricidal effect [72,81]. In cases of metastatic disease, treatment options may include streptozocin-based chemotherapy, chemotherapy with capecitabine and temozolomide, and peptide receptor radionuclide therapy (PPRT) [42]. Patients with predominant liver disease can also receive liver directed therapies such as debulking surgery, embolization, chemoembolization, radioembolization, or RFA [42,56]. Newer treatment options include sunitinib (Sutent- a tyrosine kinase inhibitor) or everolimus (Afinitor- an inhibitor of mammalian target of racamycin) [42]. Sunitinib is relatively contraindicated in patients with uncontrolled hypertension or cardiovascular disease [63]. Everolimus use is discouraged in patients with advanced diabetes [63]. Sunitinib can be used in patients with advanced pancreatic NETs; temozolomide- or streptozocin-based chemotherapy is also typically reserved for this population [56]. Poorly differentiated NETs are typically treated with first-line platinum-based chemotherapy or with salvage therapy consisting of several other chemotherapy regimens (agents such as capecitabine and telmozolomide) [56,72]. PPRT (using agents such as 177Lu-DOTATATE/DOTATOC) can have value in the treatment of well-differentiated NETs arising from the midgut [56].
NETs (well-differentiated NET's are also known as carcinoid tumors) can arise anywhere, but the GI tract is the most common site for NETs because it has the largest reservoir of neuroendocrine cells in the body [27]. GI tract NETs account for 67% of tumors (the second most common site is the tracheobronchial/lung tree accounting for about 25% of cases) [23,55]. In the GI tract, up to 30% of NETs occur in the distal part of the ileum, followed by the rectum (21-27%), and the appendix (17-20%) [23]. Less common sites include the stomach (6-9%) and the duodenum and jejunum (2-3%) [23]. The colon is an uncommon site for GI NET's [23]. Recently, it has been suggested that the rectum is actually the most common site for GI carcinoids accounting for 34% of tumors, followed by the small bowel (26%), stomach (12%), colon (8%), duodenum (8%), cecum (6%), and appendix (6%) [27].
Small bowel/mid-gut:
Midgut neuroendocrine tumors arise in the jejunum, ileum, and proximal colon [33]. The ileum is the most common site for small bowel carcinoids (NETs) [27]. Ileal NETs are usually sporadic and multiple in 26-30% of cases [23]. As a general rule, mid-gut tumors are highly prone to metastasize (to mesenteric lymph nodes, often with desmoplastic features, and liver [81]) but are generally slow growing and have a tendency to produce serotonin and other hormones resulting in carcinoid syndrome [63]. Hepatic metastases are present in 20% of cases at the time of diagnosis [23], but the disease can run an indolent course with a survival rate of more than 60%, even in patients with disseminated liver metastases [33]. Studies have shown a reduction in morbidity and mortality if the primary lesion is resected, even when metastatic disease is present (as resection can help to prevent local complications such as small bowel obstruction and vascular occlusion) [33]. Local growth factors released by the tumor (vascular endothelial growth factor, prostaglandins, and kinins) produce local fibrosis that results in desmoplasia of the surrounding mesentery [33]. The tumor may also be associated with a second primary metachronous malignancy such as prostate (26%), breast 14%), colon (9%), lung (6%), or bladder (5%) [27]. Patients with a previous small bowel malignancy are reported to have 11 times higher risk of developing a small bowel carcinoid [27].
Carcinoid syndrome is more common in small bowel NETs than at other sites in the GI system [27]. Carcinoid syndrome (Hedinger syndrome) can be found in 6-30% of patients (other authors indicate 11-50% of patients [27,33]), and hepatic metastases are present in 95% of these cases [23]. For small bowel NETs, there is a correlation between lesion size and the likelihood for metastases [23]. The incidence of mets is 15-25% for tumors smaller than 1 cm, 58-80% for tumors 1-2 cm, and over 75% for tumors greater than 2cm [23].
The primary tumor usually presents as a hyperenhancing polypoid or plaque-like mass, generally less than 2 cm in size [27]. Bowel kinking, tethering, and angulation occur because of an intense desmoplastic reaction that is thought to occur as a result of serotonin and other vasoactive peptides produced by the tumor which may damage mesenteric vessels resulting in intestinal ischemia and bowel wall thickening [27].
Stomach: Gastric NETs account for about 12% of GI NETs, are mostly composed of enterochromaffin-like cells, and three forms have been described [23,27].
Type I (70-80% of cases) is the most common type and is usually seen in middle aged women [27]. These tumors are associated with chronic atrophic gastritis and hypergastrinemia.and are usually located in the gastric fundus [23,27]. The majority of these tumors (77%) are small (measuring less than 1 cm), are non-invasive (91% submucosal), and metastasize infrequently (less than 10%) [27]. The lesions usually appear as multiple, small (<1-2cm), submucosal enhancing polyps in the gastric body and fundus [23,27]. The five year survival is 95% [27].
Type II: This is the least common type of gastric NET (carcinoid) accounting for 5-10% [27]. The lesions are usually small and multicentric [27]. Type II gastric NETs are associated with Zollinger-Ellison syndrome [27]. Nodal and liver metastases develop in 10-30% [27]. The five year survival is 60-75% [27].
Type III: Type III gastric NETs account for 15-25% of lesions, are sporadic, not associated with hypegastrinemia, and typically present as a large (>2cm) mass in the gastric body or fundus [23,27]. The lesion is not associated with atrophic gastritis, ZE syndrome, or hypergastrinemia [27]. They have a higher proliferative index (Ki-67 > 2%) compared to other subtypes and are more aggressive lesions [27]. Metastatic disease to regional lymph nodes and liver can be seen in 50-70% of patients with well-differentiated tumors and up to 100% of patients with poorly differentiated lesions [23].
Duodenum: Duodenal NETs are rare (2-8%) and are usually located in the upper portion of the duodenum [23,27]. Unlike jejunal and ileal NETs, duodenal NETs rarely arise from enterochromaffin cells [27]. Gastrin cell (G-cell) tumors account for 65% of duodenal NETs and one-third are functional (gastrinomas) [23]. G-cell duodenal gastrinomas may either be sporadic or associated with MEN-1 sundrome [27]. Those associated with MEN 1 are generally multiple and are commonly seen in the proximal duodenum [27]. The tumor can also arise from somatostatin-producing D-cells (resulting in duodenal somatostatinomas) that are predominantly seen in the periampullary region and have a unique association with neurofibromatosis type I (up to 50% may be associated with NF1 and are more frequent in African American women) [27]. On CT, these tumors may present as intralumenal polyps or mural masses showing intense enhancement or as circumferential wall thickening [27].
Appendix: Contrary to previous reports, appendiceal carcinoids are not the most common GI NET accounting for about 6% (up to 20%) of gastroenteropancreatic NETs [23,27]. However, they are still the most common tumors of the appendix (60% of cases) [27]. The lesion is typically small (<1cm) and about 70% of cases are discovered incidentally at appendectomy [23]. Histologically there are two types: the classic type and the goblet cell variety [27]. Goblet-cell NETs are more aggressive [27]. The 5-year overall survival is 88% with localized disease, 78% with regional mets, and 25% with distant mets [27].
Colon: Colon NETs are rare, but are commonly poorly differentiated [23]. The tumors tend to be larger (over 2 cm) and more frequently involve the cecum and ascending colon [27]. Most lesions have nodal or distant mets at presentation and the prognosis is generally poor [27].
Rectum: Rectal carcinoids account for 21-27% (up to 34% [27) of all gastroenteropancreatic NETs and the incidence has increased over the past three decades [23]. The lesion is often aggressive and not hormonally functioning [81]. The lesion is typically discovered incidentally at colonoscopy and the lesion is localized in 80% of cases [23]. Lesions smaller than 2cm without invasion of the muscularis propria have a very low risk of metastasis (2% versus 48% if the muscularis is invaded) [23]. Rectal pain and rectal bleeding can be found in 11-50% of patients [27].
Esophagus: Esophageal NETs are exceedingly rare (<1%) and are believed to arise from the endocrine or stem cells of the esophageal mucosa glands [27].
Pancreatic NETs account for 7% of all gastroenteropancreatic neuroendocrine tumors [23]. Although most pancreatic neuroendocrine tumors occur sporadically, as many as 25% can present in patients with genetic syndromes [24]. Non-functional tumors account for the majority of pancreatic NETs (60-80%) and are generally large (mean 4 cm) at the time of diagnosis [23,24]. Approximately 50-90% of non-functioning pancreatic NETs are malignant, but with varying biologic aggressiveness and metastatic risk [24]. Pancreatic neuroendocrine tumors generally show more aggressive clinical behavior than other forms of NET [43,81]. Up to 41.5% of pancreatic neuroendocrine tumors may not show arterial enhancement at CECT [52].
Functioning tumors are generally small (1-2 cm) [23]. Insulinomas are the most common functioning islet cell tumor (NET) followed by gastrinomas [23]. Somatostatin receptor imaging ranges from 75-100% (for nonfunctioning lesions, gastrinomas, and glucagonomas), but is lower for insulinomas because they insufficiently express somatostatin receptors (sensitivity 50-60%) [23]. Therefore, SSTR PET may not be useful for detection of insulinoma [76]. Other agents such as 68Ga-NOTA-exendin-4, which is a agonist for glucagon-like peptide-1 receptor that has a high incidence (>90%) and high expression on the cell surfaces of benign insulinomas, can be used for tumor localization [46].
Neuroendocrine tumor with unknown primary:
A significant percentage of patients (20-50%) present with metastatic disease from NET, but the primary tumor site remains undetected by conventional imaging [31,32]. The location of the primary tumor is an important factor in patient management (possible resection) and prognosis/overall survival [31,32]. In-111 Octreotide can detect the primary tumor (unrecognized on conventional imaging) in 39% of patients [31]. 68Ga-DOTANOC imaging can detect the primary in up to 59-60% of patients [31]. In another study, 68Ga-DOTATOC imaging detected the primary tumor in 38% of patients after conventional imaging failed to detect the primary lesion [53]. In a meta-analysis 68Ga-DOTATOC had a success rate of 44% for identifying the primary lesion in patients with extensive metastatic disease [54]. A prospective trial of patients with metastatic gastroenteropancreatic NETs and unknown primary demonstrated even better results with a detection rate of 95% for 68Ga-DOTATATE imaging, 46% for CT/MRI, and 31% for In-111 Octreotide [55]. 18F-DOPA can detect the primary tumor in up to 44% of cases with unknown primary [31].
Overall, the detection of specific tumors varies among the radiopharmaceuticals [31]. 18F-DOPA seems to have better diagnostic accuracy than other agents for medullary thyroid cancer, catecholamine producing tumors with low-aggressiveness, and well-differentiated carcinoid tumors of the midgut (tumors with low proliferative indexes, high levels of serotonin, urinary 5-hydroxyindoleacetic acid, metastatic lesions with the presence of CDX-2 [suggestive of a midgut primary), catecholamine derivatives, or calcitonin) [31].
18F-FDG has better accuracy for poorly differentiated NETs (lesions with a high Ki-67 of more than 15%) [31]. 68Ga-DOTA of In-111 SRS can be used for tumors with a lower proliferation index, metastatic lesions with TTF-1 (NETs of pulmonary origin) or PDX-1 (gastroduodenal or pancreatic NETs), nonfunctioning NETs, and gastroenteropancreatic NETs of hindgut origin [31].
18F-FDG:
18F-FDG PET is less sensitive than Octreotide for the evaluation of neuroendocrine tumors (NET's) [14]. This is because most well-differentiated tumors do not demonstrate significant FDG uptake[14]. In one prospective study, the overall sensitivity of 18F-FDG PET was 58% [14]. However, a feature of poorly differentiated neuroendocrine tumors is the absence or loss of somatostatin receptor activity so that although these tumors cannot be reliably images with Pentetreotide, they are frequently FDG positive [31].
For tumors with a proliferation index greater than 15, the sensitivity of FDG imaging becomes greater than Octreotide (92% versus 69%) [14]. In another study of patients with neuroendocrine tumors, FDG PET was positive in 40% of patients with a Ki67 of 2%, in 70% of patients with a Ki67 of 2-15%, and in 93% with a Ki67 of more than 15% [35]. Also important to note, is that patients with neuroendocrine tumors and negative FDG imaging have better progression-free and overall survival [35,36]. Because of tumor heterogeneity (or undifferentiated tumor clones), between 21-40% of patients with metastatic low proliferative index lesions (Ki67 <2%) and positive In-111 octreotide imaging and can also have positive FDG PET imaging (and an associated worse prognosis) [36].
Complementary 68Ga-DOTATATE / FDG PET imaging can have an impact on patient therapeutic management in up to 59% of cases [37].
One study has suggested that FDG imaging is especially useful for risk stratification, can separate G1 and G2 tumors into low and high-risk prognostic groups, and that it is superior to histologic grading (which can suffer from sampling error and an inability to identify tumor phenotype heterogeneity) [66]. In one study, FDG PET positive patients had a 5 year overall survival of 35%, versus 79% for FDG negative patients [66]. FDG positive patients had a five year progression free rate of only 18%, compared to 49% for the FDG negative patients [66]. No more than about 40% of patients with G1 disease are thought to have FDG avid lesions, whereas almost all patients with G3 disease have FDG uptake [69]. Currently, FDG is used for staging G3 disease and can be used to complement SSTR PET when the Ki-67 is 10% or more [69].
18F-fluorodihydroxyphenylalanine (18F-DOPA):
18F-fluorodihydroxyphenylalanine (18F-DOPA an analog of L-DOPA) is an agent being explored for imaging of neuroendocrine tumors [1,31]. The agents use is based on the capacity of neuroendocrine tumor cells to take up, decarboxylate, and store amino acids such as the dopamine precursor dihydroxyphenylalanine (DOPA) [3,31]. 18F-DOPA is transported into neuroendocrine cells via the sodium independent system L, in which a large neutral amino acid transporter (LAT1) protein linked to the glycoprotein CD98 is the principle mediator [9]. Amino acid decarboxylase (AADC) is important for intracellular retnetion of the agent, however, storage in neurosecretory granules is not a prerequisite for retention of the agent in gastroenteropancreatic NET's [9].
The signal-to-background ratio is, improved by inhibition of peripheral AADC activity with advanced administration of oral carbidopa (200 mg orally 60-90 minutes before tracer injection) [9,80]. Carbidopa acts by inhibiting the peripheral conversion of 18F-DOPA into 18F-fluorodopamine the bioavailability of the native tracer for tumor cells increases and uptake in surrounding tissues such as the pancreas and kidneys decreases [9]. However, carbidopa can result in negativization of focal pancreatic hot spots in patients with hyperinsulimemic hypoglycemia [32].
Dosing used for the exam has been variable- with fixed doses of 100 MBq to higher doses based upon body weight (between 3-5mBq/kg) [5,80]. In patients with extensive hepatic metastases, the bolus administration of 18F-DOPA may rarely produce a carcinoid crisis [2]. The tracer should be administered slowly, and intravenous somatostatin analogs and perhaps also ketanserin should be available to treat this condition [2,5]. Imaging can be initiated between 30 and 90 minutes following tracer administration [5].
The agent has demonstrated superior performance for lesion identification compared to somatostatin receptor scintigraphy (overall sensitivity for 18F-DOPA about 85% compared to 58% for SRS) [1]. However, the agents sensitivity and accuracy for carcinoid tumors (65-96% and 89%, respectively) is significantly better than for non-carcinoid tumors (sensitivity about 50% [8]) [3,5]. It is also recognized that the sensitivity of 18F-DOPA is higher for midgut (small intestine) NETs than for other sites and has been shown to be superior to DOTATOC imaging for imaging small intestine NETs [32,70].
Another study also suggested that 18F-DOPA imaging is superior to F-DOTATOC for the detection of subdiaphragmatic nodal mets in patients with small-intestine neuroendocrine tumors and also performed better for the detection of lesions in the liver [75]. 18F-DOPA has been shown to be superior to CT in the detection of skeletal metastases [1]. For islet cell tumors, 18F-FDOPA appears to be less sensitive than somatostatin receptor scintigraphy and endoscopic US (which has reported sensitivities of 82% and can detect lesions as small as 2-3 mm) [182].
18F-DOPA exam findings can alter patient management in up to 30% of cases (18F-DOPA results can affect management in up to 50% of patients NET's of the carcinoid type, 16% of patients with occult NET's, and 13% of noncarcinoid NET's) [1,8].
In one study of patients with metastatic mid-gut neuroendocrine tumor, an SUVpeak tumor/liver ratio of more than 4.2 was associated with significantly shorter survival (74% vs 95%) [80]. It is presumed that higher FDOPA uptake may reflect higher metabolism and biological aggressiveness of NET cells [80].
68Ga-DOTA-peptides:
68Ga is a generator produced positron emitter [40]. 68Ga (88% positron emission) has a half-life of 68 minutes and has a maximum positron energy of 1.899 MeV [21]. The effective radiation dose is 2.1 mSv per 100 MBq [40].
There are six different somatostatin receptors - 1, 2A, 2B, 3, 4, and 5 [29]. Somatostatin receptor over-expression is a feature of NET's- SSR2 and SSR5 are expressed in 70-90% of NET's [15,25]. Gastrinomas, ileal carcinoids, and VIPomas express SSR2 or SSR5 with an incidence of almost 100% [25]. Several different 68Ga-DOTA-peptides bind to somatostatin recptors and have been used for the evaluation of neuroendocrine tumors [7,11,13]. These agents have been shown to have a higher sensitivity for the detection of well-differentiated NET's (those with low Ki-67 expression) compared to conventional imaging and somatostatin receptor scintigraphy [13,19,48]. 68Ga-DOTA-Tyr3-octreotide (68Ga-DOTATOC), 68Ga-DOTA-Tyr3-Octreotate (68Ga-DOTATATE- which has received approval from the FDA for NET imaging [49]), and 68Ga-DOTA-[1-naI3]-Octreotide (68Ga-DOTANOC) are agents that specifically bind to somatostatin receptors [7,11,13].
All of the tracers bind to SSR2 (the predominant receptor in neuroendocrine tumors); DOTATOC and DOTANOC also bind to SSR5 and DOTANOC has additional good affinity for SSR3 receptors [13,55]. Of the three agents, DOTATATE has the highest affinity for the SSR2 receptor (100 times higher than In-111 pentetreotide [62]) which tends to be most over-expressed by neuroendocrine tumors [40]. The in vitro binding of DOTATATE to the SSR2 receptor is about 10-fold higher than that of DOTATOC, however, this does not seem to affect the diagnostic accuracy of the agent [20]. In fact, tumor uptake SUVmax measurements for DOTATOC have been shown to be higher than DOTATATE, although there is wide variability within patients [20]. In general, tumor uptake and SUV will decrease with increasing tumor grade (dedifferentiation) [25].
DOTATOC High affinity for SSTR2 and SSTR5 DOTATATE High affinity for SSTR2 DOTANOC High affinity for SSTR2, SSTR3, and SSTR5
The normal biodistribution for 68Ga-DOTATATE is to SSR2 receptor rich organs and include the spleen and splenules (which demonstrates the highest physiologic tracer uptake- autoradiology and immunohistochemistry studies have shown that SSR receptors in the spleen are predominantly found in the red pulp [71], adrenal glands, kidneys and urinary bladder (excretion of the hydrophilic compound occurs almost exclusively via the kidneys due to glomerular filtration and proximal tubule reabsorption; additionally, SSTRs are expressed widely throughout the distal nephron and collecting tubules in the kidneys [62]), and pituitary gland [40,55]. Moderate uptake is seen in the liver (SSRs are predominantly found in the bile ducts [71]), salivary/parotid glands, and thyroid gland [40]. There is variable activity in the stomach, small and large bowel, and prostate [40,55]. Prominent pancreatic uncinate process uptake can be seen in up to one-third of patients and should not be interpreted as pathologic [40]. This uptake is thought to be caused by a higher concentration of pancratic polypeptide producing cells in this region [76]. Gallbladder activity can be seen rarely [40]. Areas of osteoblastic activity will also demonstrate tracer uptake since osteoblasts express SSR2 receptors [40,76]. Degenerative bone disease, fractures, fibrous dysplasia, epiphyseal growth plates in children, and vertebral hemangiomas all demonstrate uptake [40,76]. Inflammatory process contain leukocytes and macrophages which express SSR2 receptors and can show uptake (infection, sarcoid, and post-XRT inflammation) [76], but the intensity is usually low grade [40].
Patient preparation-
Effect of somatostatin analogues: Some authors advocate discontinuing short acting somatostatin analog therapy for 1 to 3 days before the exam, and long acting agents 3-6 weeks prior to the exam due to the potential for competitive inhibition at the SSTR subtype 2 binding receptors [18,62,76]. The SNMMI procedure guidelines recommend patients discontinue all short-acting SSAs 12 hours prior to imaging and the EANM procedure guidelines suggest an interval of 3-4 weeks after administration of long acting SSAs to avoid potential SSTR blockade [76].
Many centers scan patients at the end of the SSA treatment cycle, if possible (i.e.- before the patients subsequent long acting SSA injection) [55,76]. Some guidelines have suggested scheduling imaging just before the next dose (which is given at 28 day intervals) [57]. However, other authors have found that even treatment with long acting somatostatin analogs does not reduce 68Ga-DOTATATE uptake to a significant degree [19] or compromise exam interpretation [57]. Data also suggests that there is upregulation of SSR in tumor cells after administration of chemotherapy [72].
Although overall SUVmax has been reported to be lower for patients on somatostatin therapy, there is also decreased tracer concentration in the liver, spleen, and thyroid in these patients and this may enhance lesion conspicuity [19,57,72]. Other authors indicate that changes in SSR intensity can be seen after the introduction of therapy with somatostatin analogues [72]. This likely reflects altered biodistribution whereby the tumor uptake may increase and appear more intense, typically associated with a decrease in uptake at physiologic sites such as the thyroid, liver, and spleen [72]. Improved lesion detection or SUV alteration should therefore be interpreted in conjunction with corroborative findings on anatomic imaging [72].
Administered activity:
For 68Ga-DOTATATE the prescribed activity is 2 MBq/kg body weight (0.054 mCi/kg) up to 200 MBq (5.4 mCi) [76]. For 68Ga-DOTATOC the adult dose is 148 MBq (4 mCi) with a range of 111-185 MBq (3-5 mCi) based on FDA prescribing information [76]. 68Ga-DOTATOC has a separate weight-based dosing for pediatric patients of 1.59 MBq/kg (0.043 mCi/kg) with a range of 11.1 MBq (0.3 mCi) to 111 MBq (3 mCi) [76].
Imaging is typically begun 45-60 minutes after the IV administration of 120-222 MBq (2 MBq (0.054 mCi)/kg body weight- approximately 4-6 mCi) of the agent [13,55]. The agents are rapidly cleared from the blood and maximal tumor activity is reached at 70 +/- 20 minutes after injection [55]. Fasting is not required for the exam [18].
The effective radiation dose to 75 kg adult following a 5 mCi injection of the agent is about 4.8 mSv [55]. Other authors report an effective radiation dose of 3.2 mSv for a 150 MBq (4.1 mCi) dose of 68Ga-DOTATATE and 3.1 mSv for a 148 MBq (4 mCi) dose of 68Ga-DOTATOC [76]. Total bone dose has been reported to be 4.8 mSv for DOTATATE and 4.3 mSv for DOTATOC [56]. The estimated dose per megabecquerel of 111In-DTPA-octreotide is approximately 3-5 times higher than for the 68Ga-labeled somatostatin analogs (the 68 minute half-life results in lower radiation exposure) [26,40]. Also- the inferior spatial and contrast resolution of planar and SPECT imaging, and the need for imaging at two time points (out to 24 hours post injection) are important drawbacks of 111In-DTPA-octreotide imaging [26]. The critical organ is the spleen, followed by the urinary bladder wall and kidneys [55]. The effective dose from the low dose CT component of the exam is about 9 mSv for an 80 mA study and closer to 1 mSv for a 10 mA CT exam [55].
Semiquantitative analysis: Lesion uptake is characterized using the modified Krennin score which is based on the lesion with the highest SSTR uptake [76]. 0= no uptake; 1= very low uptake; 2= uptake less than or equal to the liver; 3= uptake greater than the liver; and 4= uptake greater than the spleen. In general- the higher the uptake on SSTR PET, the better the expected response to PRRT therapy [76].
Sensitivity/Specificity: 68Ga-DOTATOC has been shown to be superior to conventional CT imaging and octreotide SPECT imaging for the evaluation of neuroendocrine tumors with a sensitivity of 96-97%, specificity of 92%, and an accuracy of 94-96% [8,15,45,62]. A metanalysis found an overall sensitivity of 92-93% and a specificity of 82% [54]. 68Ga-DOTATOC imaging can provide further clinically relevant information in up to 14% of patients compared to SPECT, and up to 21% of patients compared to conventional CT imaging [8]. Uptake does not appear to be influenced by the functional status of the tumor [11,12], although other authors suggest non-functional tumors may be more likely to be negative on SSTR PET than functional NETs [76].
In one study that evaluated patients with known or suspected neuroendocrine tumors, DOTATATE imaging had a sensitivity of 97%, a specificity of 95%, a PPV of 98.5%, a NPV of 90%, and an accuracy of 97% [44]. A meta-analysis found DOTATATE to have an estimated sensitivity of 91% and a specificity of 91% [47]. DOTATATE scans tend to be more positive in patients with mid gut tumors, and less sensitive for lesions in the lung, pancreas, or unknown primary [44].
As with other neuroendcrine agents, imaging typically demonstrates high uptake in well-differentiated or low-grade lesions, while tumor dedifferentiation results in decreased or absent tumor somatostatin receptor expression and false negative imaging on 68Ga-DOTATOC and 68Ga-DOTATATE [7,8,10,22,44,69]. Up to 70% of high-grade NET lesions are 68Ga-DOTATATE negative because of low, or even absent, SSR expression [49]. However, other authors note that SSR-positive disease has been observed in over 80% of grade 3 NETs and in approximately 40% of cases of neuroendocrine carcinoma [72]. Dedifferentiated NETs demonstrate FDG uptake and this is associated with a worse prognosis [8]. No more than about 40% of patients with G1 disease are thought to have FDG avid lesions, whereas almost all patients with G3 disease have FDG uptake [69]. Currently, FDG is used for staging G3 disease and can be used to complement SSTR PET when the Ki-67 is 10% or more [69]. Ultimately, a combination of SSTR and FDG PET will provide a synergistic profile of the individual patients tumor which can be used for determining when to use PRRT versus alternative therapy [69].
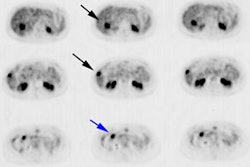
For 68Ga-DOTANOC has also been shown to be superior to conventional imaging with a reported sensitivity and specificity are 78% and 92.5%, respectively for the detection of the primary tumor, and 97% and 100% for metastases [18]. The agent can detect occult primary sites in the abdomen in up to 59% of patients, compared to 39% for SSR imaging, and 20% for CT [23]. Additionally, in one study, NOC imaging was shown to have a higher lesion based sensitivity (93.5%) compared to TATE (85.5%) and this was primarily related to higher detection of liver metastases (possibly related to lower background hepatic uptake of the tracer) and pancreatic NETS [25]. However, NOC detection of bone metastases is worse than for TATE, likely due to higher physiologic marrow activity [25].
False-positive exams may occur with all three agents due to high physiologic tracer uptake in the uncinate process/head of the pancreas [18,25,34]. False positive exams can also be seen in association with sites of inflammation or infection (macrophages also express SSTR subtype 2 receptors) including radiation pneumonitis, gastritis, reactive lymphadenopathy, and areas of recent surgery [22,49,55].
Somatostatin analogue treatment is given to slow or stop the progression of the disease, rather than provide tumoricidal effect [72]. Changes in SSR intensity can be seen after introduction of therapy with somatostatin analogues (SSAs) [72]. This reflects altered biodistribution whereby the tumor uptake may increase and appear more intense, typically associated with a decrease in uptake at physiologic sites such as the thyroid, liver, and spleen [72].
Detection of adrenal lesions with 68Ga-DOTATATE can be challenging because of marked physiologic uptake in this organ [34,49]. Other reported sites of physiologic tracer uptake include the spleen (including splenules and splenosis), liver, kidneys, pituitary, small reactive lymph nodes (possibly related to increased SSTR2 expression on activitated lymphocytes), prostate (up to 45% of patients and can be either diffuse and focal), uterus, breasts (benign uptake is typically symmetric, bilateral and mild to moderate in intensity), lungs (associated with interstitial lung disease or inflammation), brown fat, musculoskeletal system (associated with degenerative changes/osteoblastic activity and fibrous dysplasia), hemangioma (due to SSR expression), oropharynx, pineal body, thymus, aortic plaques, genitalia (likely related to SSTR3 and 5 expression in normal testes), surgical bed, and subcutaneous granulomas [38,49].
Prognosis and effect on management: In one study using 68Ga-DOTANOC, higher tracer accumulation was associated with a better prognosis (stable disease or partial therapy response), longer progression-free survival, and overall survival [11,79]. Similarly, higher SUV max on DOTATATE imaging has also been shown to be associated with a a higher response rate to peptide receptor radionuclide treatment [16,26] and a better outcome [43]. A lower SUVmax on 68Ga-DOTA-SST imaging has been shown to be associated with a worse prognosis [73]. The total somatostatin receptor expressing tumor volume has also been shown to be associated with prognosis and a volume of more than 39.1 cm3 correlates with a shorter median survival (lower total tumor volume is associated with longer progression-free survival and overall survival [73,79]. Prognosis is also dependent on tumor grade (with decreasing survival for higher grade/G3 lesions) and the presence of bone metastases is also associated with an overall worse survival [44]. .
The results of 68Ga-DOTANOC imaging can affect therapeutic management in 19-36% (up to 48% of patients [48]) and result in a modification of stage in 29% of patients [13,18]. In another study using 68Ga-DOTATATE PET and evaluating patients with negative or equivocal findings on 111In-DTPA-octreotide imaging, 68Ga-DOTATATE PET identified 74% of sites of disease and changed management in 71% of patients [15]. In another study, 68Ga-DOTATATE PET exam findings revealed unexpected metastases in 43% of patients and changed management in 60% of patients [39]. In other studies, the results of the DOTATATE exam changed management in 36-41% of patients [44,45]. In one review, 68Ga-somatostatin receptor PET imaging changed management in 39% of patients that had undergone previous 111In-Octreotide imaging [51]. The agent can also aid in the identification of patients that would be suitable for 90Y-DOTATATE or 177Lu-DOTATATE radiotherapy [15,41].
For the evaluation of bronchial carcinoids, in one study, 68Ga-DOTATATE was superior to FDG in the evaluation of well-differentiated bronchial carcinoids and was correctly able to delineate endobronchial tumor from adjacent post-obstructive consolidation [10]. In that same study, tumors of higher grade typically demonstrated high FDG uptake, and more than half were negative or demonstrated only minimal uptake on 68Ga-DOTATATE imaging [10].
Therapy response: 68Ga-DOTATATE uptake is proportional to SSTR expression may be used to monitor the effectiveness of treatment (including peptide receptor radionuclide therapy) in patients with neuroendocrine tumors [16,26,62]. Lower 68Ga-DOTA uptake and increased FDG accumulation are associated with decreased PRRT response and decreased survival.
However, changes in tumor metrics such as SUV at 68Ga-DOTATOC PET/CT during PRRT have not been found to reliably correlate with patient outcome [50,71]. A decrease in the tumor-to-spleen SUV ratio has been associated with a longer progression free survival [16]. The tumor to spleen SUV ratio appears to be a better method for monitoring tumor response as a decrease in SUV max is not necessarily associated with a good prognosis [16]. This may be because DOTATATE uptake reflects the degree of differentiation of the tumor and less uptake may indicate tumor dedifferentiation [16].
For patients with suspected neuroendocrine tumors based on tumor markers:
NET recurrence is seen in a high proportion of patients- recurrent pancreatic NET can be seen in up to 42% of patients, and recurrent small bowel NET can be seen in up to 59% of patients [30]. Elevated levels of chromogranin A (CgA) are commonly found in patients with neuroendocrine tumors (about 70-85% of patients) [22], but normal levels can be found in up to 30-40% of patients with NETs [55]. CgA can also be elevated for other reasons including treatment with proton pump inhibitors, atrophic gastritis, cardiac disease, and renal insufficiency [22,55]. 68Ga-DOTATATE imaging has been studied for the detection of neuroendocrine tumors in patients with symptoms or tumor markers suggestive of an NET [22]. The agent has a sensitivity of of 91%, a specificity of 90%, a PPV of 81%, a NPV of 90%, and an accuracy of 87% [22]. False positive findings can be seen in association with sites of inflammation [22].
In another study, 68Ga-DOTATATE had a sensitivity of 90%, specificity of 82%, PPV 81%, NPV 90%, and accuracy of 86% for the evaluation of post operative tumor recurrence [30]. False positive results can be seen in association with inflammatory change [30]. False negative exams can occur in patients with small volume disease, small lung nodules, or more dedifferentiated tumors [30].
Other agents for imaging neuroendocrine tumors:
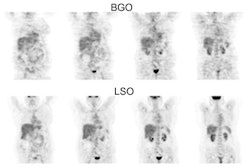
64Cu-DOTATATE: 64Cu (17% positron emission) has a half-life of 12.7 hours, a maximum positron energy of 0.633 MeV, average energy of 278 keV, a short mean positron range of 0.6 mm, and a resolution loss from the positron range of 0.2 mm in FWHM (the high positron energy of 68Ga - max 1.899 MeV and average energy of 836 keV- results in a mean positron range of 3.5 mm and a spatial resolution loss of 0.8 mm at FWHM which limits the spatial resolution) [21,41,69,76]. As a result of its greater positron range, is estimated that there will be an additional 10-20% underestimation of quantification for small lesions (10-15 mm) with 68Ga compared to 64Cu [76]. The agent can be produced as a once-daily batch with a shelf life of more than 24 hours (the short half life of 68Ga - 68 minutes- limits its availability) [41].
The typical dose is 148 MBq (4 mCi) [76]. The longer half life results in higher target-to-background ratios on delayed imaging and simplifies distribution and imaging logistics [69,76]. Overall- in comparison to 68Ga the agent has a longer half-life, lower radiation dose, and a lower positron range which provides superior spatial resolution [67]. However, the positron yield for 64Cu is only 17.4 %, which is significantly lower than the 88.9% yield of 68Ga, which results in the need for longer scan times- approximately 2 times longer, and the need for a smoother spatial filter to reduce image noise [76].
The radiation dose per injected activity for 64Cu-DOTATATE is also 20-25% higher than for 68Ga-DOTATATE- 4.7 mSv for a 148 MBq (4 mCi) dose compared to 3.2 mSv [76]. Overall, 64Cu-DOTATATE produces diagnostic scans that are equivalent to those of 68Ga-labeled compounds [76].
The agent has also been developed for neuroendocrine tumor imaging [21]. The agent has been shown to be superior to 111In-DTPA-Octreotide imaging (sensitivity 97% and specificity 97%; detecting lesions in up to 36% of cases not evident on 111In-DTPA-Octreotide imaging) with an overall lower radiation dose to the patient (6.3 mSv) [41].
Somoatostatin Receptor Antagonists:
Unlike SSTR agonist agents, SSTR antagonists are not internalized by the cells- despite this fact, they typically display higher occupancy (SSTR antagonists interact with a larger variety of SSTR conformations which allows binding of both activated and inactivated SSTRs), more prolonged binding, and have higher affinity for SSR receptors compared to agonists and also have lower uptake in normal organs [59,60,68,77,78]. SSTR antagonists are more chemically stable and hydrophobic than SSTR agonists, with a consequent longer duration of action and stabilization in a lipid-rich environment [78].
SSTR antagonists also have rapid blood pool clearance [69]. The higher tumor-to-background for SSR antagonists can result in a higher detection of liver metastases and an overall significantly higher lesion-based sensitivity compared to Ga-68 DOTATOC (94% vs 59%) [60,69].
REFERENCES:
(1) J Nucl Med 2004; Becherer A, et al. Imaging of advanced neuroendocrine tumors with 18F-fluoro-DOPA PET. 45: 1161-1167
(2) J Nucl Med 2005; Koopmans KP, et al. Carcinoid crisis after injection of 6-18F-fluorodihydroxyphenylalanine in a patient with metastatic carcinoid. 46: 1240-1243
(3) J Nucl Med 2006; Montravers F, et al. Can fluorodihydroxyphenylalanine PET replace somoatostatin receptor scintigraphy in patients with digestive endocrine tumors? 47: 1455-1462
(4) Radiographics 2007; Intenzo CM, et al. Scintigraphic imaging of body neuroendocrine tumors. 27: 1355-1369
(5) J Nucl Med 2008; Jager PL, et al. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. 49: 573-586
(6) J Nucl Med 2008; Garin E, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. 50: 858-864
(7) J Nucl Med 2009; Putzer D, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. 50: 1214-1221
(8) J Nucl Med 2009; Gabriel M, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. 50: 1427-1434
(9) J Nucl Med 2009; Minn H, et al. 18F-DOPA: a multiple-target molecule. 50: 1915-1918
(10) J Nucl Med 2009; Kayani I, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. 50: 1927-1932
(11) J Nucl Med 2010; Campana D, et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in enuroendocrine tumors. 51: 353-359
(12) J Nucl Med 2007; Schillaci O. Somatostatin receptor imaging in patients with neuroendocrine tumors: not only SPECT? 48: 498-500
(13) J Nucl Med 2010; Ambrosini V, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. 51: 669-673
(14) J Nucl Med 2010; Binderup T, et al. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scinitgraphy, and 18F-FDG PET. 51: 704-712
(15) J Nucl Med 2010; Srirajaskanthan R, et al. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. 51: 875-882
(16) J Nucl Med 2010; Haug AR, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. 51: 1349-1356
(17) J Nucl Med 2011; Ruf J, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: spotlight on the CT phases of a triple-phase protocol. 52: 697-704
(18) AJR 2011; Naswa N, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. 197: 1221-1228
(19) J Nucl Med 2011; Haug AR, et al. Treatment with Octreotide does not reduce tumor uptake of 68Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. 52: 1679-1683
(20) J Nucl Med 2011; Poeppel TD, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imagign of neuroendocrine tumors. 52: 1864-1870
(21) J Nucl Med 2012; Pfeifer A, et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. 53: 1207-1215
(22) J Nucl Med 2012; Haug AR, et al. The role of 68Ga-DOTATATE PET/CT in suspected neuroendocrine tumors. 53: 1686-1692
(23) Radiology 2012; Sahani DV, et al. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. 266: 38-61
(24) AJR 2013; Gallotti A, et al. Incidetnal neuroendocrine tumors of the pancreas: MDCT findings and features of malignancy. 200: 355-362
(25) J Nucl Med 2013; Wild D, et al. Comparison of 68Ga-DOTANOC versus 68Ga-DOTATATE PET/CT with gastroenteropancreatic neuroendocrine tumors. 54: 364-372
(26) J Nucl Med 2013; Walker RC, et al. Measured human dosimetry of 68Ga-DOTATATE. 54: 855-860
(27) AJR 2013; Geneshan D, et al. Imaging features of carcinoid tumors of the gastrointestinal tract. 201: 773-786
(28) AJR 2013; Kim KW, et al. Update on the magaement of gastroenteropancreatic neuroendocrine tumors with emphasis on the role of imaging. 201: 811-824
(29) AJR 2013; Sharma P, et al. Somatostatin receptor-based PET/CT of intracranial tumors: a potential area of application for 68Ga-DOTA peptides. 201: 1340-1347
(30) Radiology 2014; Haug AR, et al. Neuroendocrine tumor recurrence: diagnosis with 68Ga-DOTATATE PET/CT. 270: 517-525
(31) J Nucl Med 2014; Schillaci O. 18F-DOPA and other radiopharmaceuticals for imaging unknown primary neuroendocrine tumors. 55: 357-359
(32) J Nucl Med 2014; Imperiale A, et al. 18F-fluorodihydroxyphenylalanine PET/CT in patients with neuroendocrine tumors of unknown origin: relation to tumor origin and differentiation. 55: 367-372
(33) Radiographics 2014; Woodbridge LR, et al. Midgut neuroendocrine tumors: imaging assessment for surgical resection. 34: 413-426
(34) J Nucl Med 2014; Etchebehere EC, et al. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-Octreotide SPECT/CT, and Whole-Body MR Imaging in Detection of Neuroendocrine Tumors: A Prospective Trial. 55: 1598-1604
(35) J Nucl Med 2014; Schillaci O. Can PET/CT guide personalized treatment of patients with gastroenteropancreatic neuroendocrine neoplasms. 55: 1757-1758
(36) J Nucl Med 2014; Bahri H, et al. High prognostic value of 18FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long term evaluation. 55: 1786-1790
(37) J Nucl Med 2014; Simsek DH, et al. Can complimentary 68Ga-DOTATATE and 18F-FDG PET establish the missing link between histopathology and therapeutic approach in gastroenteropancreatic neuroendocrine tumors? 55: 1811-1817
(38) AJR 2014; Kagna O, et al. Neuroendocrine tumor imaging with 68Ga-DOTA-NOC: physiologic and benign variants. 203: 1317-1323
(39) J Nucl Med 2015; Herrmann K, et al. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: the referring physicians perspective. 56: 70-75
(40) Radiographics 2015; Hofman MS, et al. Somatostatin receptor imaging with 68Ga-DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. 35: 500-516
(41) J Nucl Med 2015; Pfeifer A, et al. 64Cu-DOTATATE PET for neuroendocrine tumors: a prospective head-to-head comparison with 111In-DTPA-Octreotide in 112 patients. 56: 847-854
(42) J Nucl Med 2015; van Vliet EI, et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3] octreotate. 56: 1647-1653
(43) J Nucl Med 2015; Ambrosini V, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. 56: 1843-1848
(44) J Nucl Med 2016; Skoura E, et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: experience from a national referral center in the United Kingdom. 57: 34-40
(45) J Nucl Med 2016; Deppen SA, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. 57: 708-714
(46) J Nucl Med 2016; Luo Y, et al. Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-exendin-4 for detecting localized insulinoma: a prospective cohort study. 57: 715-720
(47) J Nucl Med 2016; Deppen SA, et al. 68Ga-DOTATATE compared with 111In-DTPA-Octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. 57: 872-878
(48) J Nucl Med 2017; Panagiotidis E, et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with neuroendocrine tumors. 58: 91-96
(49) J Nucl Med 2017; Fendler WP, et al. 68Ga-DOTATATE PET/CT interobserver agreement for neuroendocrine tumor assessment: results of a prospective study on 50 patients. 58: 307-311
(50) J Nucl Med 2017; Ilan E, et al. Parametric net influx rate images of 68Ga-DOTATOC and 68Ga-DOTATATE: quantitative accuracy and improved image contrast. 58: 744-749
(51) J Nucl Med 2017; Barrio M, et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. 58: 756-761
(52) Radiology 2017; Jeon SK, et al. Nonhypervascular pancreatic neuroendocrine tumors: differential diagnosis frmo pancreatic ductal adenocarcinomas at MR imaging- retrospective cross-sectional study. 284: 77-87
(53) J Nucl Med 2017; Menda Y, et al. Localization of unknown primary site with 68Ga-DOTATOC PET/CT in patients with metastatic neuroendocrine tumor. 58: 1054-1057
(54) J Nucl Med 2017; Graham MM, et al. 68Ga-DOTATOC imaging of neuroendocrine tumors: a systematic review. 58: 1452-1458
(55) J Nucl Med 2017; Bodei L, et al. Current concepts in 68Ga-DOTATATE imaging of neuroendocrine neoplasms: interpretation, biodistribution, dosimetry, and molecular strategies. 58: 1718-1726
(56) J Nucl Med 2018; Hope TA, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. 59: 66-74
(57) J Nucl Med 2018; Ayati N, et al. Long-acting somatostatin analog therapy differentially alters 68Ga-DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. 59: 223-227
(58) Radiographics 2018; Ganeshan D, et al. Tumors in von Hippel-Lindau syndrome: from head to toe- comprehensive state-of-the-art review. 38: 849-866
(59) J Nucl Med 2018; Bodei L, Weber WA. Somoatostatin receptor imaging of neuroendocrine tumors: from agonists to antagonists. 59: 907-908
(60) J Nucl Med 2018; Nicolas GP, et al. Safety, biodistribution, ad radiation dosimetry of 68Ga-OPS202 in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase I imaging study. 59: 909-914
(61) J Nucl Med 2018; Nicolas GP, et al.Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastropancreatic neuroendocrine tumors: a prospective phase II imaging study. 59: 915-921
(62) AJR 2018; Sanli Y, et al. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATE PET/CT. 211: 267-277
(63) J Nucl Med 2019; Mohamed A, Strosberg JR. Medical management of gastropancreatic neuroendocrine tumors: current strategies and future advances. 60: 721-727
(64) AJR 2019; Kendi AT, et al. Therapy with 177Lu-DOTATATE: clinical implementation and impact on care of patients with neuroendocrine tumors. 213: 309-317
(65) J Nucl Med 2020; Adams LC, et al. Quantitative 3D assessment of 68Ga-DOTATOC PET/MRI with diffusion-weighted imaging to assess imaging markers for gastroenteropancreatic neuroendocrine tumors: preliminary results. 61: 1021-1027
(66) J Nucl Med 2021; Binderup T, et al. 18F-FDG PET is superior to WHO grading as a prognostic tool in neuroendocrine neoplasms and useful in guiding PRRT: a prospective 10-year follow-up study. 62: 808-815
(67) AJR 2021; Sheikhbahaei S, et al. Neuroendocrine tumor theranostics: an update and emerging applications in clinical practice. 217: 495-506
(68) J Nucl Med 2021; Baum RP, et al. First-in-human study of SSTR antagonist 177Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with meastatic neuroendocrine neoplasms: dosimetry, safety, and efficacy. 62: 1571-1581
(69) J Nucl Med 2021; Park S, et al. Somatostatin receptor imaging and theranostics: current practice and future prospects. 62: 1323-1329
(70) J Nucl Med 2021; Imperiale A, et al. Imaging of small intestinal neuroendocrine neoplasms: is SSTR PET the holy grail? 62: 1347-1348
(71) J Nucl Med 2021; Ortega C, et al. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. 62: 1406-1414
(72) AJR 2022; Galgano SJ, et al. Imaging of neuroendocrine neoplasms: monitoring treatment response- AJR expert panel narrative review. 218: 767-780
(73) J Nucl Med 2022; Thuillier P, et al. Prognostic value of whole-body PET volumetric parameters extracted from 68Ga-DOTATOC PET/CTin well-differentiated neuroendocrine tumors. 63: 1014-1020
(74) J Nucl Med 2022; Jha A, et al. Choice is good at times: the emergence of [64Cu] 68Cu-DOTATATE-based somatostatin receptor imaging in the era of [68Ga] 68Ga-DOTATATE. 63: 1300-1301
(75) J Nucl Med 2022; Ouvrard E, et al. 18F-DOPA PET/CT at the forefront of initial or presurgical evaluation of small-intestine neuroendocrine tumors. 63: 1865-1870
(76) J Nucl Med 2022; Hope TA, et al. SNMMI procedure standard/EANM practice guideline for SSTR PET: imaging neuroendocrine tumors. 64: 204-210
(77) J Nucl Med 2023; Hindie E, et al. The latest advances in peptide receptor radionuclide therapy for gastroenteropancreatic neuroendocrine tumors. 64: 522-524
(78) J Nucl Med 2023; Imperiale A, et al. The emergence of somatostatin antagonist-based theranostics: paving the road toward another success? 64: 682-684
(79) AJR 2023; Navin PJ, et al. Imaging of small-bowel neuroendocrine neoplasms: AJR expert panel narrative review. 221: 289-301
(80) J Nucl Med 2023; De Rycke O, et al. High tumor uptake on 18F-FDOPA PET/CT indicates poor prognosis in patients with metastatic midgut neuroendocrine tumors: a study from the groupe d'etude des tumeurs endocrines and ENDOCAN-RENATEN network. 64: 1699-1705
(81) J Nucl Med 2024; Strosberg Jr, et al. Sequencing of somatostatin-receptor-based therapies in neuroendocrine tumor patients. 65: 340-348