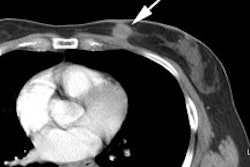
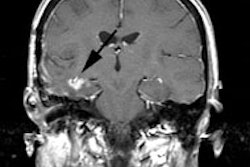
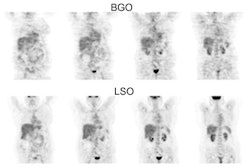
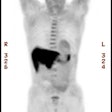
PET Oncologic Imaging:
[18]F-fluorodeoxyglucose (FDG) Imaging:
One of the biochemical characteristics of malignant cells in an enhanced rate of glucose metabolism due to increased numbers of glucose transporter proteins (in particular Glut-1) and increased intracellular enzyme levels of hexokinase and phosphofructokinase which promote glycolysis [4,16]. This enhanced glycolytic rate of malignant cells facilitates their detection utilizing PET FDG imaging. FDG enters the cells by the same facilitated transport mechanism as glucose and is intracellularly phosphorylated by hexokinase into FDG-6-phosphate. FDG-6-phosphate does not enter into further metabolism and accumulates intracellularly [4]. The signal derived from tumors represents an average of the FDG uptake throughout the lesion [16]. In vitro studies have shown that FDG uptake may be determined mainly by the number of viable tumor cells within a lesion [16]. Non-tumoral tissue such as necrotic and fibrotic tissue may reduce tracer uptake [16]. The bottomline is that FDG accumulation within a tumor is likely related to a complex interaction between the cellular energy demand and the tumoral microenvironment [7]. One drawback of PET is the underestimation of metabolic activity in tumors that are smaller than two times the spatial resolution of the scanner (partial volume effect) [6]- however, using modern PET scanners, objects that measure as small as 5 mm can be visualized [1].
Unfortunately, FDG is not a cancer specific agent and its uptake has been described in a number of inflammatory lesions including sarcoid, tuberculosis, fungal infection, and cerebral abscess [1]. The increased accumulation is probably related to a markedly increased rate of glycolysis within activated inflammatory cells. Assessment for malignancy and metastatic disease with PET FDG imaging is improved when supplemented by CT images [2]. PET imaging can also identify a more accessible tumor site for histologic confirmation.
FDG imaging is performed in the fasting state to minimize competitive inhibition of FDG uptake by glucose [14]. A 4 hour fast is recommended prior to initiation of the PET FDG study. A 12 hour fast may decrease accumulation by the myocardium and improve detection of mediastinal metastases in cases of lung or breast cancer. If there is a possibility of an elevated serum glucose level, a serum glucose level is obtained prior to FDG administration. FDG uptake is significantly influenced by plasma glucose levels and uptake will be decreased when plasma glucose levels are elevated (elevated serum glucose levels can result in decreased FDG accumulation within the tumors) [4,14]. If the glucose level is higher than 150-200 mg/dL, the study should be delayed until the glucose level is under 200 mg/dL [3]. Administering insulin at the same time as FDG should be avoided because it tends to increase accumulation in skeletal muscle and thus less FDG is available for accumulation in tumors [3]. In patients with diabetes, blood sugar control should be achieved with oral hypoglycemic agents or insulin (not administered near the time of FDG administration) [3].
FDG PET scans are performed approximately 60 minutes following the intravenous injection of 10 to 20 mCi of FDG and usually require about 1 hour to acquire [15]. Some centers acquire a 4 minute brain scan about 30 minutes following FDG administration using the three-dimensional acquisition mode [3]. The three-dimensional mode is performed with the septa removed and provides an approximately 4 fold increase in sensitivity for the detection of annihilation radiation [3]. There is an increase in scatter in the three-dimensional mode compared with the two-dimensional mode acquisition, but the improved sensitivity provides better image quality [3]. Scatter radiation in the body is greater than in the head, and three-dimensional imaging is generally not used for body imaging [3]. For the body, a two dimensional acquisition is acquired from the base of the brain to the mid thighs [3].
PET technology allows for attenuation correction of the images. This can produce a more accurate final image that may detect smaller lesions, especially when they are deep within the body [15]. The disadvantages of attenuation correction is that it requires more time for image acquisition and there is the potential to add noise to the image if the attenuation measurements become misaligned by patient motion [15].
The normal distribution for FDG includes:
1- The cortex of the brain- there is generally very intense tracer uptake in the brain because the brains only energy source is glucose.
2- Myocardium- in the post-prandial state, there is marked cardiac activity. Little myocardial activity is generally noted in the fasting state, however, uptake is variable.
3- Renal/Urinary bladder- due to renal excretion of the agent. Hydration and frequent voiding promote diuresis and help to decrease the radiation dose to the genitourinary tract [4].
4- Liver- faint, heterogeneous activity is common. It is possible for both liver metastases and treated liver metastases to have liver-equivalent activity- as a result, such lesions cannot be reliably identified by PET imaging [17].
5- Muscular activity- if exercised. Note: Muscles of mastication or larynx can accumulate tracer if eating or talking [4]. Hyperventilation may induce uptake in the diaphragm and stress-induced muscle tension is often seen in the trapezius and paraspinal muscles [4].
6- Gastrointestinal tract- variable activity. Cecal uptake may be prominent [4] and the stomach is usually faintly seen (but uptake can be intense) [4]. There can be normal mild FDG activity in the esophagus possibly due to swallowed saliva or smooth muscle metabolism and this can potentially obscure subtle lesions [11]. Esophagitis in the distal esophagus is also a common cause of tracer accumulation [4]. There is usually uptake in the lymphoid tissue of Waldeyer's ring [4].
7- Thymic uptake can be present in children and in patients with thymic hyperplasia (seen in up to 16% of patients) following chemotherapy [4,7,8]. Thymic enlargement can persist for up to 6 months following completion of therapy [7]. Thymic activity is usually "V" shaped and generally not very intense.
8- Bone marrow- faint activity is generally identified within the bone marrow [7]. The accumulation is generally homogeneous and has SUV ratios between 0.7 to 1.3 [7]. Increased bone marrow activity can be seen with bone marrow recovery following chemotherapy, but this usually resolves by one month post-therapy [4]. Treatment with granulocyte stimulating factor can also produce diffuse skeletal FDG accumulation [9]. In an animal model following XRT, the irradiated bone marrow shows a significant increase in FDG accumulation over baseline on day 1, a significant decrease on day 9, and a return to normal between 18 and 30 days [12,13].
9- Thyroid- thyroid uptake can occur in association with thyroiditis or Graves' disease. Focal thyroid uptake can occur with autonomously functioning thyroid nodules and thyroid malignancies.
10- Lymph nodes- nodal uptake can occur if the agent extravasates into the soft tissues at the site of injection (always inject in arm opposite primary lesion) [4]
11- Gonads- male gonadal activity can be seen and is quite variable
12- Degenerative joint disease and degenerative disk disease can be associated with increased tracer accumulation
13- Vascular activity- in scans not corrected for transmission, vascular activity in the large vessels in the thighs and pelvis can be seen in about 80% of patients [10].
14- Breasts- variable uptake within glandular tissue
Other agents used for oncologic imaging:
Amino Acids:
Amino acid transport is generally increased in malignant cells and amino acid imaging is less influenced by inflammation [18]. The replacement of a carbon atom by 11C does not chemically change the molecule [18]. Amino acid agents typically have low CNS uptake which provides good contrast with tumor uptake (compared to FDG which has significant CNS activity) [18]. Images are usually performed within the first hour after tracer administration and patients should be fasting prior to the exam [18].
11C-L-methylmethionine measure amino acid uptake/protein synthesis (tumor cells require an external supply of methionine). It has primarily been sued for imaging of CNS neoplasms [18]. There is low uptake in the brain, and somewhat higher uptake in the salivary glands, lacrimal glands, bone marrow, and occasionally the myocardium [18]. Abdominal uptake in the liver and pancreas (usually high uptake) is frequently seen, and there is variable intestinal uptake of the agent [18]. Moderate activity is seen in the renal cortex [18]. Pituitary uptake is high [18].
Other amino acid agents include: C-11 thymidine is used in the evaluation of tumor DNA replication and cell division rates. 68Ga-EDTA has been used in the evaluation of disruption of the blood brain barrier. C-11 choline is an agent that is incorporated into tumor cells by conversion into C11-phosphorlycholine which is trapped inside the cell. This is followed by synthesis of 11C-phosphatidylcholine which constitutes a main component of cell membranes. Because tumor cells duplicate very quickly, the biosynthesis of cell membranes is also very fast. Consequently the uptake of C-11 choline in tumors represents the rate of tumor cell proliferation [5].
REFERENCES:
(1) J Nucl Med 1994; Larson SM. Cancer or inflammation? A holy grail for nuclear medicine.
35 (10): 1653-55 (No abstract available)
(2) AJR 1998; Eubank WB, et al. Imaging of oncologic patients: Benefit of combined CT and FDG PET in the diagnosis of malignancy
(3) J Nucl Med 1999; Coleman RE. PET in lung cancer. 40: 814-820
(4) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(5) J Nucl Med 2000; Hara T, et al. Sensitive detection of mediastinal lymph node metastasis of lung cancer with 11C-Choline PET. 41: 1507-1513
(6) Radiology 1997; Moog F, et al. Lymphoma: role of whole-body 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. 203: 795-800
(7) Blood 1999; Jerusalem BG, et al. Whole-body positron emission tomography using 18-F-Fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical conventional tomography scan imaging. 94 (2): 429-433
(8) Acta Oncol 1999; Bangerter M, et al. Positron emission tomography with 18-Fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. 38 (6): 799-804
(9) J Nucl Med 1999; Moog F, et al. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. 40 (9): 1407-13
(10) The normal PET scan. von Schulthess GK, et al. In- Clinical positron emission tomography. Correlation with morphological cross-sectional imaging. Ed. von Schulthess GK. Lippincott Williams & Wilkins. 2001: 49-69.
(11) Radiographics 2000; Skehan S, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. 20: 713-723
(12) J Nucl Med 2000; Higashi T, et al. Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: Preclinical PET studies. 41: 2026-2035
(13) J Nucl Med 2000; Ballinger JR. Local and distant effects of radiotherapy on FDG accumulation in bone marrow. 41: 2036-37
(14) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(15) Ann Thorac Surg 1998; Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. 65: 1821-29
(16) J Nucl Med 2001; Avril N, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. 42: 9-16
(17) J Nucl Med 2001; Dimitrakopoulou-Strauss A, et al. Quantitative PET studies in pretreated melanoma patients: A comparison of 6-[18F] Fluoro-L-Dopa with 18F-FDG and 15O-water using compartment and noncompartment analysis. 42: 248-256
(18) J Nucl Med 2001; Jager PL, et al. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. 42: 432-445
Breast Tumors:
Mammography and breast ultrasound are the modalities primarily used to screen for breast cancer. Mammography is highly sensitive and can identify 80-90% of patients with breast cancer [2]. Unfortunately, a positive mammographic finding does not always equate with malignancy. Only between 2 to 4 of 10 patients with abnormal mammograms are found to have breast cancer at histologic analysis [2]. Additionally, about 10% of breast cancers cannot be identified mammographically even when they are palpable [2]. Invasive lobular carcinoma can be difficult to identify mammographically because of its diffuse growth pattern and tendency to have an opacity equal to or less than the surrounding parenchyma [2]. Ultrasound can permit rapid differentiation of cystic and solid lesions, but accurate differentiation between benign and malignant solid lesions is sometimes difficult [2].
Breast cancers display a considerable variation in FDG uptake [7]. Invasive ductal carcinoma exhibits significantly higher FDG uptake compared to invasive lobular carcinoma [7]. Focal nodular lesions also show higher accumulation when compared to infiltrative/diffuse lesions [7]. Additionally, the greater the degree of tumor proliferation and the greater the degree of tumor dedifferentiation, the greater the FDG accumulation [7]. The estrogen and progesterone status of a lesion do not appear to influence FDG uptake [7].
Focal breast lesions:
PET imaging using 18-FDG has demonstrated potential in the differentiation of benign from malignant breast lesions. One of the benefits of PET imaging is that it is not affected by the density of breast tissue. Image quality is also not impaired by prior surgery, prior XRT, or breast implants [2]. Reported sensitivities for FDG PET range from 64% to 96%, and specificity's from 94% to 100% [1,2,4]. The primary limitation of FDG-PET imaging for evaluation of breast carcinoma is its inability to detect small lesions (under 1 cm in size) due to partial volume effects and natural tracer accumulation in the breast which may obscure low uptake lesions [2]. Sensitivity is higher for lesions greater than 1 to 2 cm in size, but even larger lesions may not demonstrate tracer accumulation (up to 20% of lesions between 2 to 5 cm in size may be falsely negative on PET FDG imaging) [2]. Additionally, a larger percentage of invasive lobular carcinomas are not detected (65% in one study), possibly due to low tumor cell density, its infiltrative nature, and low metabolic activity [2]. None-the-less, PET imaging may be useful in a carefully selected subgroup of patients with inconclusive conventional imaging procedures. For lesions of sufficient size, a positive PET scan carries a high positive predictive value that the lesion is breast cancer [2]. False-positive findings are also infrequent (about 5% of benign lesions will demonstrate tracer uptake) [2]. False positive exams have been reported in association with inflammation, fibroadenomas (about 10% accumulate FDG [2]), and ductal adenomas [2]. Specialized PET mammographic imaging systems may hold future promise [8,9].
Staging:
FDG PET imaging can also aid in the detection of metastases to lymph nodes and other unsuspected sites in the body. Pre-operative determination of node status is essential as clinical evaluation is usually inaccurate. Up to 40% of patients with a clinically negative exam demonstrate the presence of mets at histologic examination. Conversely, 40% of patients suspected of having lymph node mets, are found to be negative on histologic review [1]. Reported sensitivities for locoregional nodal metastases range from 50% to 100% [2-5]. Lower sensitivities for the detection of axillary nodal mets can be expected for lymph nodes smaller than 1 cm in diameter [4] and consequently, a negative FDG PET exam should not preclude lymphoscintigraphy and axillary node dissection [4].
Additionally, the estrogen receptor status of breast cancers can be reliably evaluated with PET using the radiolabeled estrogen analog F-18 estradiol (FES). Furthermore, FES accumulation is a receptor mediated process that can be blocked with anti-estrogen therapy [1].
Monitoring response to therapy:
Fifteen to 25% of breast cancers are large (over 3 cm) or locally advanced (T3,T4, or TxN2) at the time of initial presentation [5]. Patients with such tumors pose major therapeutic problems due to a high incidence of locoregional recurrence after surgery and an overall poor prognosis [5]. Neoadjuvant (primary chemotherapy) is now increasingly used in the management of these patients [5]. An important advantage of primary chemotherapy is that it increases the rate of breast conserving surgery by reducing tumor volume [6]. Patients with only minimal residual disease following completion of primary chemotherapy have also been shown to have significantly higher disease free and overall survival rates compared to patients with gross residual disease [6]. More importantly, studies have demonstrated that patients with unresponsive tumors may achieve an improved survival with the use of alternative and/or more prolonged courses of chemotherapy [5]. It would be of obvious benefit if patients with unresponsive tumors could be identified earlier, thereby avoiding ineffective treatment [5]. Using clinical assessment, it is not possible to accurately predict which patients have responded to therapy and which patients have not [6]. Imaging metabolic pathways offers an alternative method to visualize the effects of treatment [6]. Changes in tumor glucose uptake (reflected by FDG) can be used to demonstrate and guide effective treatment of breast cancer [5,6]. Decreased tracer accumulation within a lesion (decreased tumor metabolism) after chemotherapy indicates effective treatment [5,6]. Additionally, assessment of therapy response can be determined earlier with FDG PET than with any other method of conventional therapy evaluation (i.e.: before any appreciable decrease in tumor mass can be detected) [2,5,6]. FDG PET imaging after a single pulse of chemotherapy is able to predict a complete pathologic response with a sensitivity of 90-100% and a specificity of 74-85% [**,5,6]. One may hypothesize that a decrease in primary tumor and locoregional lymph node activity probably indicates a similar response in occult disseminated disease [5]. Therefore, the number of patients that develop clinically apparent metastases under ineffective chemotherapy may be reduced [6]. One limitation of FDG PET imaging is that it is not capable of differentiating patients with residual microscopic disease from those with a complete response [6]. None-the-less, FDG PET offers great promise in the early assessment of tumor response to therapy [6].
NOTE: Partial volume effects may contribute to decreased tumor uptake ratios obtained after the tumor has begun to decrease in size [5].
**NOTE: Smith et al used a using a decrease in tracer uptake ratio of 20% from the pre-treatment value to identify responders; Schelling et al used a decrease in SUR below 55% of the baseline scan [5,6].
Post-therapy changes versus tumor recurrence:
FDG PET has demonstrated usefulness in discriminating tumor recurrence from post-operative and post-radiation changes in the breast.
REFERENCES:
(1) J Nucl Med 1995; Dehdashti F, et al. Positron tomographic assessment of estrogen
receptors in breast cancer: Comparison with FDG-PET and in-vitro receptor assays. 36 (10):
1766-74
(2) J Clin Onc 2000; Avril N, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: Use and limitations. 18: 3495-3502
(3) Radiologic Clinics of North America 2000; Pisano ED, et al. Digital mammography, sestamibi breast scintigraphy, and positron emission tomography breast imaging. 38: 861-869
(4) J Comput Assist Tomogr 2000; Yutani K, et al. Comparison of FDG-PET with MIBI-SPECT in the detection of breast cancer and axillary lymph node metastasis. 24: 274-280
(5) J Clin Oncology 2000; Smith IC, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. 18: 1676-1688
(6) J Clin Oncology 2000; Schelling M, et al. Positron emission tomography using [18F] fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. 18: 1689-1695
(7) J Nucl Med 2001; Avril N, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. 42: 9-16
(8) J Nucl Med 2001; Murthy K, et al. Results of preliminary clinical trials of positron emission mammography system PEM-I: A dedicated breast imaging system producing glucose metabolic images using FDG. 41: 1851-1858
(9) J Nucl Med 2001; van Rijk PP, van Dongen AJ. Limited angle, limited approach? 41: 1859-1860 (No abstract available)
CNS Neoplasms:
Primary CNS neoplasms are uncommon (7 to 19 cases per 100,000 people) [9]. Brain metastases are up to 10 times more common than primary brain tumors and occur in 20-40% of patients with cancer [9]. A predominant biochemical feature of rapidly growing tumor cells is an ability to sustain high rates of glycolysis under anaerobic conditions. Glucose (or FDG) utilization in tumors is characterized by increased aerobic and anaerobic glucose metabolism, an increased number of glycolytic enzymes, and increased cellular glucose transport. Glucose loading may help to improve lesion detection by causing a more significant suppression of FDG uptake in normal brain tissue compared to neoplastic lesions. This failure to suppress uptake is most likely due to a lack of the normal physiologic control of glucose metabolism in neoplastic lesions [1].
Primary lesion:
PET FDG examination:
Adults receive 10 mCi of FDG injected IV in a room with low ambient light and noise. Following injection patients rest quietly with their eyes closed until imaging between 30-60 minutes after injection. Scans can be corrected for attenuation using an emission scan or with empiric uniform correction [9]. Correlation with anatomic imaging studies is essential for accurate exam interpretation. The amount of intracellular FDG is proportionate to the rate of glucose transport and intracellular phosphorylation [9]. Malignant cells generally have high rates of aerobic glucose metabolism [9]. FDG uptake within an astrocytoma has good correlation with the histologic grade of the tumor- poorly differentiated tumors (Grades III and IV) commonly exhibit increased FDG uptake, while uptake is typically only mild in low grade (Grade I-II) lesions. A few low grade tumors, such as pilocystic astrocytoma and pituitary adenoma may have high FDG uptake [9]. Some metastases may also have metabolic activity less than normal cortex [9]. Higher grade malignant lesions may also show low FDG accumulation if they have an increased reliance on anaerobic metabolism [9].
FDG imaging may also provide prognostic information. In patients with high grade lesions, those with normal or hypometabolic activity on FDG imaging had an improved one year survival (75%) compared to patients with hypermetabolic lesions (29% one year survival) [2]. On cerebral blood flow imaging, gliomas and cerebral metastases generally have variable CBF which is often mildly reduced in comparison to normal brain tissue. CBF and CBV within the lesion have not been shown to correlate with the tumor grade. Oxygen extraction and oxidative metabolism are usually markedly reduced in brain tumors, despite the heightened glucose utilization.
Other agents used for CNS malignancy imaging:
11C-L-methylmethionine (measuring amino acid uptake/protein synthesis) also demonstrates increased uptake in 80 to 90% of malignant brain tumors (tumor cells require an external supply of methionine). Uptake of the tracer seems to correlate with histologic tumor grade, with increased activity in higher grade lesions (grade III and IV), but only mild uptake in grade II tumors. C-11 methionine imaging appears to provide the greatest lesion-to-background ratios (all tumors have a ratio of much greater than 1:1). Reported sensitivity can be as high as 97% for high grade lesions, but sensitivity is much lower for low grade tumors [10].
A drawback of PET imaging is that uptake of both FDG (typically spotty) and 11C-methionine (more prominent) has been described surrounding brain hematomas on scans performed between 20 and 30 days following the event, possibly secondary to a subacute gliotic reaction about the hemorrhage. This activity will decrease and disappear over time (2 to 3 months), but initial differentiation from a neoplasm that has bled may be difficult (between 2-14% of spontaneous intracranial hemorrhage is secondary to tumors). Correlation with the patients contrast enhanced CT or MRI may help as C-11 methionine uptake in non-neoplastic bleeds will be concordant with the contrast enhancement identified about the abnormality. Increased tracer accumulation extending beyond the enhanced area on CT/MRI is indicative of a hemorrhage associated with an underlying CNS neoplasm [3,4]. C-11 methionine uptake has also been reported in brain abscesses [5] and sites of radiation necrosis [6].
Lymphoma versus toxoplasmosis in HIV:
FDG PET has also been used to differentiate lymphoma from toxoplasmosis in patients with AIDS [8].
Differentiation of recurrent tumor from radiation necrosis:
The general approach to treatment of brain neoplasms is surgical resection of solitary lesions or limited disease, followed by radiation therapy (with or without chemotherapy) [9]. Solitary lesions may alternatively be treated with local field radiotherapy or stereotactic radiosurgery, while multiple or metastatic lesions receive whole-brain radiation [9]. Radiation injury occurs in 5-37% of cases [9]. Radiation injury to the brain is the major dose-limiting complication of radiotherapy [9]. Although the term "radiation necrosis" is used to describe radiation injury, it is inaccurate because pathologically radiation injury is not limited to necrosis [9]. The incidence of radiation injury depends on the total dose and rate of delivery (fractionalization) [9]. Very young children are more susceptible than adults and chemotherapy (adriamycin and methotrexate) can potentiate radiation injury [9].
Radiation injury can be divided into acute, early-delayed, and late-delayed stages [9]. Late-delayed radiation injury occurs in 5-37% of cases, months to years (10 years) following XRT. About 70% of cases occur in the first 2 years after treatment [9]. The primary mechanism of late-delayed radiation injury is vascular endothelial injury or direct damage to oligodendroglia [9]. The white matter is affected more than the gray matter [9]. Edema, mass effect, and blood-brain barrier disruption are commonly associated with this condition and differentiation from recurrent tumor can be difficult on conventional imaging exams. Treatment of radiation injury range from conservative measures to control intracranial pressure to surgical excision of the edematous mass [9].
PET studies with FDG have shown that recurrent tumor exhibits hypermetabolism of glucose, while non-necrotic irradiated brain shows hypometabolism, and necrotic brain has no detectable metabolic activity [9]. False negative FDG PET exams can occur with lesions with a small or microscopic tumor volume [9]. FDG PET may be less sensitive in differentiating recurrence from radiation injury in low grade (well-differentiated) tumors due to their inherently lower metabolic activity [9]. A reversible decrease in metabolic activity in viable tumors in the immediate post radiation period may also result in a decrease in FDG accumulation [9]. Because of the usual high metabolic activity in the cerebellar hemispheres, it can be difficult to detect contrast between brain tumor recurrence and adjacent normal tissue [7]. False-positive scans may be caused by radiation injury, which activates repair mechanisms that can increase aerobic glucose metabolism [9]. Normal healing during the immediate post-surgical period (up to 3 months) may also result in a false-positive exam [9]. Other causes of focal increased glucose metabolism include seizure activity and abscesses [9]. Overall, FDG PET has a sensitivity of 81-86%, and a specificity of 50-94% in the differentiation of recurrent tumor from radiation brain injury [9]. One added benefit of FDG PET imaging is in selection of the best site for biopsy of a focal lesion [9].
REFERENCES:
(1) J Nucl Med 1994; Ishizu K, et al. Effects of hyperglycemia on FDG uptake in human
brain and glioma. 35 (7): 1104-09
(2) Cancer 1988, 62:1074-78
(3) J Nucl Med 1994; Dethy S, et al. Carbon-11-methionine and fluorine-18-FDG PET study in brain hematoma. 35 (7) 1162-66
(4) J Nucl Med 1995; Ogawa T, et al. Carbon-11-methionine PET evaluation of intracerebral hematoma: Distinguishing neoplastic from non-neoplastic hematoma. 36 (12): 2175-79
(5) J Comput Assist Tomogr 1993; Ishii K, et al. High L-methyl-[11C] methionine uptake in brain abscess: A PET study. 17: 660-1 (No abstract available)
(6) Acta Radiol 1991; Ogawa T, et al. Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. 32: 197-202
(7) J Nucl Med 1995; 36 (1): 163
(8) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(9) J Nucl Med 2001; Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. 41: 1861-1867
(10) J Nucl Med 2001; Jager PL, et al. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. 42: 432-445
Colon cancer:
General/Background:
Colorectal carcinoma is a major health problem with 130,000 to 165,000 new cases diagnosed each year in the United States [3,11]. It is the second leading cause of death from cancer in the United States and Europe [6]. At the time of diagnosis, colorectal carcinoma is localized in only 36% of patients; regional lymph node metastases are present in 39% and distant mets are present in 19% [2]. In patients with rectal carcinoma, because the hemorrhoidal vein drains into the IVC, pulmonary metastases can occur without hepatic mets. Up to 15-20% of patients with colorectal carcinoma will have liver metastases at the time of initial surgery. Surgical resection of the primary tumor and the associated liver metastases is the only possible chance for cure [11]. Liver resection for hepatic metastases is considered only for patients with one to 4 mets confined to one lobe of the liver, and when no other adenopathy or metastases are evident (i.e.: isolated intrahepatic metastases) [3]. Up to 5% of patients demonstrate synchronous colonic carcinomas, although synchronous adenomatous polyps are found in 30% of cases [4]. About 5% of patients with colorectal carcinoma develop metachronous carcinomas at a later point in time [4].
In patients with colorectal carcinoma the expectation of cure depends on the stage of the initial tumor. Assessment of tumor extent (within and beyond the bowel wall) and the presence or absence of adenopathy are paramount for determining prognosis and the risk for tumor recurrence [3]. Primary tumors confined to the submucosa are cured in more than 90% of cases [2] and the risk for tumor recurrence is only 5% [3]. The risk for recurrence increases to 10% if the tumor invades the muscularis propria, but remains confined to the bowel wall (T2 tumor) [3]. Tumors that have extended beyond the bowel wall without lymph node involvement are cured in 60-80% of cases [5] and recurrence is seen in about 25% of cases [3]. Recurrence is seen in about 50% of cases in which the tumor has extended into neighboring structures [3]. If one to 3 lymph nodes are positive the five year survival drops to 66%, and is only 37% if four or more nodes are positive [5]. Post-operative development of hepatic metastases is reported to occur in up to 30% of patients within 2 years of curative surgery [5].
Recurrent colorectal carcinoma occurs in 37-44% of patients within 2 years of curative resection [5]. Early detection of potentially resectable metastases or tumor recurrence can lead to improved survival [2]. Serum levels of carcinoembryonic antigen (CEA) may be used to monitor for the presence of recurrences with a sensitivity of 59%, and a specificity of 84% [2]. Unfortunately, the CEA test cannot localize the site of recurrent disease. Local recurrence at the surgical site accounts of 19-45% of cases, whereas distant mets account for 25-44% [5,7]. Locoregional recurrence is more commonly encountered with rectal carcinoma, while colon carcinoma recurrence is more commonly seen in the liver or abdomen [6]. Between 15-20% of patients with locally recurrent disease are amenable to curative resection [7]. However, long term survival after attempted curative resection for metastatic or locally recurrent tumor is only about 35%- most likely due to undetected metastatic disease at other sites. Appropriate selection of patients most likely to benefit from curative surgery is crucial in order to avoid unnecessary surgery that may cause major morbidity [10].
The role of FDG PET imaging:
FDG PET imaging is best used to more accurately stage disease before attempted curative resection or to confirm equivocal findings on conventional imaging studies before initiating treatment [10]. PET imaging is not useful for the assessment of small mucosal or submucosal tumors [1]. PET imaging is clearly superior to anti-CEA imaging for the detection of distant metastases (liver, bone, and lung) and lymph node involvement when evaluating for recurrent disease [2].
Indications for FDG PET imaging in colorectal carcinoma include (presently reimbursed):
1- A rising CEA level in the absence of a known source
2- Equivocal lesion on conventional imaging (Evaluation for recurrent tumor for indicators other than rising CEA [i.e.: Abnormal CT scan finding])
3- Detection of hepatic and extrahepatic metastases in primary staging
4- Preoperative staging prior to resection of recurrent disease
5- Distinguishing local recurrence from post-operative scar
Evaluation for recurrence and post-operative/post-therapy change:
Because it provides a whole body survey, FDG examinations can identify unsuspected sites of disease in patients being considered for curative resection of locally recurrent disease. FDG PET has very good sensitivity (89-91%) and is more accurate than CT for the detection of liver metastases [1]. FDG imaging has also been shown to be superior to conventional imaging for detecting extrahepatic metastases (sensitivity 94% versus 67% for conventional imaging) [10]. FDG PET can change patient management in 26% to 65% of cases by identifying a resectable or non-resectable metastasis that was unsuspected clinically, not seen, or equivocal on CT [1,9-11].
FDG imaging can also be used to differentiate local recurrence from scarring following radiation therapy [1,2]. Tracer accumulation within an abnormality is strongly suggestive of tumor recurrence. Absence of tracer uptake indicates lack of disease and these patients can be followed with observation [9]. FDG PET has a sensitivity of up to 90%, a positive predictive value up to 88%, and a negative predictive value of 92% for the detection of local recurrence (compared to 71% sensitivity for CT/colonoscopy) [10].
Limitations of FDG PET imaging in colorectal carcinoma:
As with other cancers, FDG PET imaging may fail to detect small volume disease [9] due to partial-volume averaging with small lesions (under 1 cm) or in necrotic lesions with only a thin rim of viable tissue [1]. FDG imaging has also been shown to be less sensitive for the detection of mucinous carcinoma, possibly due to a relative hypocellularity [10]. False-positive exams can also occur. Normal gastrointestinal tract accumulation of the tracer may be difficult to differentiate from a malignant lesion [1]. Uptake at sites of prior anastamosis has also been described [10]. All extrahepatic foci of FDG PET accumulation should be evaluated with a CT scan through the region of abnormality to confirm the presence of a lesion and evaluate its resectability [1]. Following radiation therapy, FDG accumulation may be related to radiation induced inflammation. However, by 6 months following XRT, any activity noted on PET FDG exams should be considered consistent with tumor recurrence [1].
REFERENCES:
(1) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(2) J Nucl Med 2000; Willkomm P, et al. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of recurrence of colorectal carcinoma. 41: 1657-1663
(3) Radiol Clin North Am 1997; Thoeni RF. Colorectal cancer. Radiologic staging. 35: 457-485
(4) Radiology 2000; Levine MS, et al. Diagnosis of colorectal neoplams at double-contrast barium enema examination. 216: 11-18
(5) Radiographics 2000; Horton KM, et al. Spiral CT of colon cancer: Imaging features and role in management. 20: 419-430
(6) J Am Coll Surg 2000; Lechner P, et al. Can postoperative surveillance with serial CEA immunoscintigraphy detect resectable rectal cancer recurrence and potentially improve tumor-free survival? 191: 511-518
(7) Annals of Surgery 1997; Hughes K, et al. Use of carcinoembryonic antigen radioimmunodetection and computed tomography for predicting resectability of recurrent colorectal carcinoma. 226: 621-631
(8) Clin Nucl Med 1999; Yoshoka T, et al. FDG PET evaluation of residual masses and regrowth of abdominal lymph node metastases from colon cancer compared with CT during chemotherapy. 24: 261-263
(9) Clin Pos Imag 2000; Kalff V, et al. F-18 FDG PET for suspected or confirmed regional recurrence of colon cancer: A prospective study of impact and outcome. 3: 183
(10) Dis Colon Rectum 2000; Whiteford MH, et al. Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. 53: 759-70
(11) The American Surgeon 1999; Boykin KN, et al. The use of FDG-positron emission tomography for the evaluation of colorectal metastases of the liver. 65: 1183-85
Esophageal Carcinoma:
The prevalence of esophgeal cancer has increased dramatically over the past 30 years (350-800% increase in adenocarcinoma) [1]. Early stage esophageal cancer is often asymptomatic and detection is uncommon [3]. As a consequence, esophageal cancer usually presents at an advanced stage with 75% of patients having lymph node metastases at presentation. Overall survival rate at 5-years is 10% or less [3]. Patients with lymph node metastases have a 5 year survival of only 3%, compared to 42% without nodal involvement [1]. Distant metastases are found in about 18% of patients at presentation- especially to abdominal nodes, liver, and lung. Surgery for operative candidates is not without significant risk and is associated with a mortality between 5 to 20% [1]. Proper identification of patients that are truly surgical candidates is crucial so that patients with advanced stage disease can be referred for prompt multimodality treatment protocols [3]. Although endoscopic evaluation and CT imaging form the mainstays of clinical staging, they cannot accurately identify all sites of disease [3]. FDG PET imaging holds great promise as a means to fully assess for the presence of unsuspected sites of disease in patients presenting with esophageal carcinoma.
FDG PET imaging is presently reimbursed for the following indications:
1- Pre-surgical staging and restaging of esophgeal carcinoma
2- Monitoring for recurrence (may be covered unless strong negative evidence is present)
Primary tumor:
Despite reported sensitivities between 91-100% for demonstrating primary esophageal cancer (both squamous cell and adenocarcinoma), FDG PET imaging is not useful for the evaluation of the patients primary esophageal tumor. False positive exams can occur due to esophageal inflammation and there can be normal mild FDG activity in the esophagus possibly due to swallowed saliva or smooth muscle metabolism which could potentially obscure subtle lesions [1]. PET imaging is also not sensitivity for assessing for the presence of local invasion [1].
Nodal metastases:
CT has an accuracy of 40-73% for the detection of pathologic mediastinal nodes (using a 1 cm size criteria) [1]. Accuracy for detection of left-sided gastric or celiac nodes is 79% (using a size criteria of 8 mm) [1]. The reported accuracy of FDG PET in the staging of locoregional lymph node mets varies from 24-90% [1]. As with local tumor invasion, the principal limitation of FDG PET with regard to the detection of local nodal metastases adjacent to the primary tumor is its relatively poor spatial resolution which reduces sensitivity to 24-72% [1]. Although the presence of these nodes carries prognostic information, local nodal mets do not preclude curative surgical resection (i.e.: they do not necessarily change clinical management) [1]. It is important to remember that supraclavicular, cervical, and celiac nodes are considered M1 disease [1]. The presence of pathologic adenopathy in these other sites would substantially alter patient management.
Distant Metastases:
The major advantage of FDG PET over conventional imaging is the ability to detect distant metastases [1]. In a prospective study of 100 patients with esophageal carcinoma referred for surgery, FDG PET imaging was shown to be more accurate than CT in detecting distant metastases (84% versus 63%) [4]. The presence of distant metastases has considerable impact on patient management as patients are then considered non-resectable. FDG PET has a reported sensitivity of 69%-100%, a specificity of 84%-90%, and an accuracy of 84%-91% for the evaluation of distant metastases [1,3,4]. Sensitivity of CT for distant metastases has been reported to be as low as 46% [4]. FDG PET can demonstrate metastatic disease not identified on conventional studies in about 17% to 22% of patients [1,3,4]. PET exams can also exclude metastatic disease at sites considered abnormal on conventional imaging. Metastatic lesions not identified on FDG PET exams are typically less than 1 cm in size and are usually in the lung or liver [4].
PET imaging may also provide prognostic information. When PET exams demonstrate only local disease, the 30 month survival has been reported to be up to 60%; while patients with distant disease on PET exams had a 30 month survival of 20% [4].
Recurrent Esophageal Cancer:
Even after apparently curative surgery, the overall 5 year survival for esophageal carcinoma is only 30-50% [2]. Two-thirds of patients have a recurrence within one year and nearly all within 2 years after primary operation [2]. In about one-third of patients, the recurrence is located in the operative field (to regional lymph nodes or a recurrent mass) [2]. The remainder, and majority of recurrences are distant metastases [2]. Some reports suggest that early detection and aggressive treatment of recurrent disease may result in prolonged tumor-free survival and occasionally cure [2]. FDG PET imaging allows a highly sensitive means for whole-body staging in patients with suspected recurrent esophageal carcinoma [2].
For the diagnosis of perianatomotic recurrence FDG PET had a sensitivity of 100%, specificity of 57%, and an accuracy of 74% (compared to 100%, 93%, and 96% for conventional imaging) [2]. False positive exams can be seen in association with recent (up to 2 months post procedure) endoscopic stricture dilatation [2]. For the diagnosis of regional and distant recurrences FDG PET had a sensitivity of 94%, a specificity of 82%, and an accuracy of 87% (compared to 81%, 82%, and 81% for conventional imaging) [2]. Therefore, preliminary results would suggest that FDG PET does not seem to enhance the diagnostic accuracy for locoregional recurrence [2]. However, conventional imaging studies cannot reliably differentiate post surgical change/scar from tumor recurrence. FDG PET is extremely useful in this setting [1]. By demonstrating increased metabolic activity within an abnormality detected by conventional imaging, a greater confidence in the diagnosis of recurrent disease can be achieved. Additionally, PET imaging can help to guide diagnostic biopsy to the regions of greatest metabolic activity.
For the evaluation of metastatic disease, FDG PET had a sensitivity of 95%, compared to 79% for conventional imaging [2]. PET imaging can provide additional information in 27% of patients being evaluated for recurrent esophageal carcinoma [2]. Up to 12% of patients with negative conventional imaging exams can be shown to have recurrent disease by FDG PET [2].
REFERENCES:
(1) Radiographics 2000; Skehan S, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. 20: 713-723
(2) J Thorac Cardiovasc Surg 2000; Flamen P, et al. The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. 120: 1085-92
(3) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(4) Ann Thorac Surg 1999; Luketich JD, et al. Evaluation of distant metastases in esophgeal cancer: 100 consecutive positron emission tomography scans. 68: 1133-37
Head and Neck Tumors:
In the evaluation of head and neck cancer, uptake in the muscles of mastication or laryngeal muscles can mimic metastases [4]. Therefore, it is important to keep the patient in the resting state with no eating or talking during the distribution phase following injection.
For planning which type of surgical neck dissection will be used and in determining the need for post-operative chemotherapy/XRT accurate pre-operative staging (TNM) is essential for head and neck tumors. Using PET FDG imaging, lymph node mets from squamous cell carcinoma of the mouth can be visualized with a high sensitivity (75 to 91%) and specificity (88-96%), which is superior to MRI (36-78% and 71-94% respectively). MRI is limited because pathologic nodes are often considered solely on the basis of size criteria (larger than 1 cm).
Even with PET imaging, small metastatic nodes (under 4 mm) may still go undetected. Another difficulty with PET is that differentiation of reactive nodes from malignant ones is not possible and this results in a high false-positive rate.
REFERENCES:
(1) J Nucl Med 1995; Braams JW, et al. Detection of lymph node metastases of squamous-cell
cancer of the head and neck with FDG-PET and MRI. 36: 211-16
(2) Radiology 1995; Oct.: p.205
(3) J Nucl Med 1995; Laubenbacher C, et al. Comparison of fluorine-18-fluorodeoxyglucose PET, MRI, and endoscopy for staging head and neck squamous-cell carcinomas. 36: 1747-57
(4) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
PET in Lung Cancer:
General
In Staging
Lymphoma:
Both Hodgkins and Non-Hodgkins lymphomas are amenable to curative therapy. Many of the affected patients are young with otherwise good life expectancies. In men aged 15-54 years of age, Hodgkins disease is the third leading cause of death, and non-Hodgkins lymphoma is the second leading cause of death [2]. The stage at presentation and cell type determine the patients overall prognosis and optimal method for treatment. The anatomic extent of disease is the single most important factor influencing relapse-free and total survival in patients with Hodgkins disease [2]. Unfortunately, accurate demonstration of all sites of disease can be difficult to obtain utilizing conventional staging methods. Between 20-30% of patients with presumed localized supra-diaphragmatic disease at the time of initial presentation, can be shown to have infra-diaphragmatic disease detected at staging laparotomy [5]. Laparotomy is invasive and associated with immediate and potential long term complications- especially in post-splenectomy patients. The optimal staging method for lymphoma should be able to non-invasively identify all sites of disease.
Conventional imaging with CT or MR is presently the primary means to evaluate and stage patients with lymphoma. These modalities can reveal anatomic abnormalities suggestive of tumor involvement. Conventional imaging is primarily dependent on lymph node size for detection of tumor involvement. Generally, lymph nodes greater than 1 cm in size are considered suggestive of tumor involvement (depending on anatomic location). Unfortunately, normal sized lymph nodes can harbor malignancy and enlarged nodes may be merely be reactive. Furthermore, infiltrative involvement of the liver, spleen, and bone marrow cannot be accurately detected.
Although gallium imaging has been utilized for the evaluation of lymphoma, the exam suffers from several limitations. The examination requires several days to complete (PET exams are performed about 1 hour following injection of the agent). Although probably adequate for imaging of the chest, gallium suffers from decreased sensitivity for evaluation of the abdomen due to high physiologic liver and bowl activity [1]. Due to lower anatomic and spatial resolution, gallium imaging has decreased ability to detect small lesions when compared to FDG PET imaging [1,4]. Also, negative post-therapy scans are not useful unless a pre-therapy scan demonstrating tumor accumulation of the agent were performed [7]. Post therapy scans can also suffer from non-specific hilar uptake of gallium which can be confused for recurrent disease [8].
PET imaging for lymphoma: Compared to conventional imaging, FDG PET imaging has certain advantages. FDG PET imaging provides high lesion contrast and very good anatomic localization due to the tomographic nature of the images [2]. Lesion detection is based upon a biochemical signal (increased metabolism) rather than anatomic criteria [2]. PET imaging can also provide a complete body survey which is important when evaluating a multifocal disease process such as lymphoma [2]. Two of the main trends in healthcare at the present time are increasing cost-effectiveness and decreasing the number of invasive procedures. Non-invasive, metabolic FDG PET imaging can provide additional information regarding accurate staging, reduce the number of surgical procedures required for staging, and can also monitor patient response to treatment [8]. Whole body PET imaging followed by additional conventional imaging of selected areas has been shown to be more cost effective than a standard conventional staging algorithm [4]. Total management costs were calculated to be almost half compared to the conventional staging algorithm ($66,292 for conventional staging and $36,250 for FDG PET staging) [2,4].
PET imaging with FDG has demonstrated high sensitivity for the detection of abnormal lymph nodes in patients with lymphoma and for patient staging [2]. There is a correlation between the degree of FDG uptake and the histologic grade of the lymphoma [3]- with high grade lesions demonstrating greater metabolic activity [4]. Reported sensitivities range from 62-100%, but the studies are very heterogeneous and direct comparison of results is difficult due to variations in methodology and reference data [4,8] (specificity has been reported to be 94% [8]). Despite this variability, FDG has been shown to be more accurate than CT in staging lymphoma [3]. This is because PET imaging can detect additional lesions (nodal and extra-nodal) which are not identified by convention imaging [4,5]. PET findings can result in a change in patient Stage in 8% to 16% of patients (either up-stage or down-stage) [1,4,5,10].
Nodal disease: PET imaging will generally detect all abnormal lymph nodes identified on CT [1]. Additional sites of nodal disease are commonly detected, particularly in the abdomen where CT may miss small mesenteric lymph nodes [1].
Extranodal disease: Extra-nodal organ involvement carries a worse prognosis when compared to patients with isolated nodal disease [5]. FDG PET can detect up to 57% more extranodal sites of disease than noted on CT scan [5]. Reported rates for hepatic and splenic involvement are 3.2% and 23% respectively, for Hodgkins disease and 15% and 22%, respectively, for non-Hodgkins lymphoma [5]. Infiltrative involvement of the liver, spleen, and bone marrow cannot be accurately detected on conventional imaging as organ size is a poor predictor of tumor involvement [5]. In about 30% of patients, splenic enlargement is not related to malignancy [5]. Sensitivity rates for CT range from 15-37% for splenic infiltration, and from 19% to 33% for liver infiltration [5]. Diffuse increased tracer accumulation within these organs on FDG PET imaging can confirm their involvement [5]. Infiltrative lymphomatous involvement of the spleen can be detected with a 67% increased frequency when compared to CT imaging [5].
Bone marrow involvement indicates stage IV disease and carries a less favorable prognosis for patients with non-Hodgkin's lymphoma [11]. Bone marrow involvement in newly diagnosed lymphoma occurs in 5%-15% (10%) of patients with Hodgkins disease and in 19% to 83% (25%) of patients with non-Hodgkins lymphoma [5,6,10,11]. To be detected on CT imaging, osseous involvement needs to be focal and associated with bone destruction. Patients with infiltrative marrow involvement demonstrate no bone destruction and are usually asymptomatic [5]. Both focal and infiltrative osseous lesions can be detected on FDG PET imaging [5]. Infiltrative osseous involvement appears as generalized increased tracer activity within the bone marrow [5]. FDG PET imaging has been shown to be superior to Tc-99m-MDP bone scintigraphy for the evaluation of osseous involvement by tumor [9]. FDG Imaging can detect up to 42% more osseous lesions than Tc-99m-MDP bone scintigraphy [9]. Lymphomatous involvement of the bone marrow can be detected on FDG PET with a sensitivity of 81% to 88%, and a specificity of 100% [1,6,10].
Bone marrow biopsy (bilateral posterior iliac crests) represents the standard diagnostic procedure to confirm bone marrow involvement [6]. Unfortunately, bone marrow biopsy samples only a small volume and is associated with a high rate of false negative findings (due to patchy or localized marrow involvement) which may lead to errors in patients management [6]. PET imaging can reveal osseous involvement not detected on bone marrow biopsy in 12.8% of patients [6]. This finding resulted in an increase in patient stage in 10% of patients [6]. Conversely, bone marrow biopsy can reveal tumor involvement in 5% of patients which is not detected by FDG PET imaging [6]. The cases in which FDG PET was false negative involved low or intermediate grade lymphomas with only discrete displacement of normal marrow (up to 10%) [6]. Because the degree of FDG uptake declines in a lower grade of malignancy- the combination of lower numbers of malignant cells and low FDG tumor uptake results in a low marrow activity concentration [6].
PET Limitations: False positives exams have been reported in association with non-specific inflammatory lymph nodes and could potential result in incorrect patient staging [1,2]. However, it should be pointed out that conventional imaging can also produce false positive exams when a reactive enlarged node is classified as involved with tumor. Lack of increased metabolic activity within enlarged nodes has been shown to correlate with lack of tumor involvement [4]. Small lesions (under 1 cm in size) may lack sufficient metabolic activity to be properly identified [2]. However, using this size criteria, nodes less than 1 cm in size would not be characterized as abnormal by conventional imaging either. PET imaging can be degraded by physiologic activity which can mask lesions- although to a much lesser degree than gallium scintigraphy. The brain has intense uptake of FDG and may obscure a CNS lesion. As standard staging with CT does not usually include the brain, the decreased sensitivity of FDG PET for this region is not a true limitation of the exam. With either standard or PET staging, concern for CNS involvement would require further evaluation with MR imaging. Cardiac uptake is high in a post-prandial state, and therefore patients should be fasting to minimize myocardial uptake and maximize evaluation of the chest. Renal and bladder activity (due to urinary excretion) can obscure abnormalities in the abdomen. Faint bowel activity can also be seen, but is generally not enough to cause confusion with actual disease. Bone marrow involvement may not be demonstrated in patients with lower grade lymphomas with only discrete marrow displacement (up to 10%) [6].
Other applications for PET imaging in the evaluation of lymphoma include:
1- Assess for tumor recurrence: PET provides a rapid whole body survey to assess for recurrent disease.
2- Monitor response to therapy: FDG activity reflects the mass of viable tumor cells [4]. Changes in tumor metabolism occur prior to any significant decrease in tumor mass [4]. Early data indicates that following effective chemotherapy, there is a rapid decrease in metabolic activity within the tumor (a decrease in SUV between 75-90% within 7 days of initiation of therapy) [4]. Persistent high FDG uptake several weeks after completion of chemotherapy is an indication of lack of tumor response. Early assessment of response to chemotherapy can help to avoid the toxicity and cost of ineffective treatment [4]. Second line therapy can then be instituted early to maximize the patients potential for cure [4].
3- Differentiate scar from residual tumor mass: A residual mass after treatment of lymphoma is a clinical challenge because it may represent residual viable tumor or tissue fibrosis [7]. Unfortunately, there are no reliable radiographic characteristics that permit differentiation between malignant and fibrotic tissue. FDG will accumulate in viable tumor, but does not accumulate in fibrotic or necrotic tissue [4]. In patients with mediastinal lymphoma, up to 64% of patients exhibit some persistent mediastinal radiographic abnormality following completion of therapy [4]. However, only 18% of these patients will eventually relapse [7]. FDG accumulation within a residual mass is associated with a higher predictive value for relapse and an overall worse survival compared to patients without FDG uptake [7]. Persistent metabolic activity within a residual mass should prompt strong consideration for additional therapy [7]. Although up to 25% of patients with negative PET scans and a residual mass do eventually relapse, the relapse is in another site in 80% of these cases [7].
REFERENCES:
(1) Radiology 1997; Moog F, et al. Lymphoma: role of whole-body
2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. 203: 795-800
(2) J Nucl Med 1997; Hoh CK, et al. Whole-body FDG-PET imaging for staging of Hodgkin's disease and lymphoma. 38: 343-348
(3) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(4) Clinical Positron Imaging 1998; Romer W, et al. Positron emission tomography in diagnosis and therapy monitoring of patients with lymphoma. 1 (2): 101-110
(5) Radiology 1998; Moog F, et al. Extranodal malignant lymphoma: Detection with FDG PET versus CT. 206: 475-481
(6) J Clin Oncology 1998; Moog F, et al. 18-F-Fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. 16 (2): 603-609
(7) Blood 1999; Jerusalem BG, et al. Whole-body positron emission tomography using 18-F-Fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical conventional tomography scan imaging. 94 (2): 429-433
(8) Acta Oncol 1999; Bangerter M, et al. Positron emission tomography with 18-Fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. 38 (6): 799-804
(9) J Nucl Med 1999; Moog F, et al. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. 40 (9): 1407-13
(10) Ann Oncol 1998; Bangerter M, et al. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin's disease. 9 (10): 1117-22
(11) Clin Nucl Med 2000; Lee J, et al. Dichotomy between Tc-99m MDP bone scan and Fluorine-18 fluorodeoxyglucose coincidence detection positron emission tomography in patients with non-Hodgkin's lymphoma. 25: 532-535
Melanoma:
General:
Melanoma is the most aggressive form of skin cancer. The lesion frequently spreads to regional lymph nodes once the vertical growth phase develops and is then likely to metastasize to any organ [1]. The presence of lymph node metastases is well established as a negative prognostic indicator. For early stage melanoma, patients presently undergo surgical resection of the lesion followed by examination of the sentinal node [1]. The sentinal node is the first draining node of the lymphatic bed draining the tumor- if the sentinal node is free of tumor, the remainder of the nodes in that basin are likely to be free of tumor. This approach obviates the need for a more radical lymph node dissection [1].
Presently, FDG PET is reimbursed for the following indications in patients with melanoma:
1- Evaluation of extranodal metastases at initial staging or during
follow-up after treatment
2- Evaluation for recurrent melanoma as an alternative to gallium scan
PET imaging is not reimbursed for the evaluation of regional lymph
node metastases for primary staging
FDG PET can be useful in detecting lymph node and distant metastases- particularly in high-risk patients (melanoma thickness greater than 1.5 mm - Clark's level IV) [1,3]. PET FDG imaging has been shown to be superior to conventional imaging for the detection of melanoma metastases [3]. Peripheral skin metastases as small as 3 mm have been detected [3]. PET FDG has been shown to have a sensitivity of 95%, a specificity of 84%, and an accuracy of 91% in the detection of metastases to involved lymph node basins [2]. The detection of nodal metastases is dependent upon the size of the metastasis [2]. Detection can be as high as 100% for metastases greater than 1 cm in size, while detection of metastases smaller than 5 mm is only 23% [2]. False positive exams can occur [2].
REFERENCES:
(1) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(2) J Nucl Med 2000; Crippa F, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. 41: 1491-1494
(3) Cancer 1998; Rinne D, et al. Primary staging and follow-up of high risk melanoma patients with whole-body 18-F-fluorodeoxyglucose positron emission tomography: Results of a prospective study of 100 patients. 82: 1664-71
Musculoskeletal Tumors:
FDG PET can be used to evaluate musculoskeletal tumors. FDG exams can detect distant metastases, evaluate for recurrence, and monitor response to therapy. Unfortunately, PET FDG false negative examinations have been reported in association with malignant lesions- particularly chondrosarcomas and plasmacytomas [1]. Also, increased FDG uptake in a skeletal lesion is not pathognomonic for malignant neoplasms; it has also been described with benign bone lesions (non-ossifying fibroma, fibrous dysplasia, giant cell tumors, eosinophilic granuloma, and aneurysmal bone cyst) and inflammation/infection.
Perhaps, one of the more important applications of FDG PET is in evaluation of response to therapy. Chemotherapy and radiation can be used pre-operatively to decrease tumor size in order to facilitate the limited resection needed for limb-salvage surgery [2]. The correlation between histopathologic tumor necrosis after preoperative chemotherapy with clinical prognosis is well established [2]. Functional imaging with FDG PET allows detection of biochemical changes within the lesion prior to visible morphological changes. A decrease in the tumor to non-tumor uptake ration of greater than 30% after chemotherapy has been shown to correlate well with extensive histologic tumor necrosis [2]. For patients that fail to demonstrate a response to treatment, early therapy modification can be performed. This is important as post-operative modifications in therapy regimens for non-responders have not been shown to improve patient outcome [2].
FDG PET imaging is superior to bone scintigraphy for evaluation of tumor response to therapy as bone healing (sclerosis) will result in increased Tc-99m-accumulation ("flare" phenomenon) [2].
Specific tumors:
Osteosarcoma:
Combined Fluorine-18 (blood flow and osteoblastic response) and FDG (metabolism) imaging can be combined to evaluate primary bone neoplasms. In patients with osteosarcoma, increased uptake of both agents is identified in a distribution corresponding to the bone and soft tissue components of the tumor respectively. In general, there is good correlation between the amount of FDG uptake and the histologic grade of the lesion. Following successful treatment of the lesion, F-18 uptake will still be seen in areas corresponding to sclerosis, but regional accumulation of FDG in these areas will be normal.
REFERENCES:
(1) J Nucl Med 2000; Schulte M, et al. Grading of tumors and tumorlike lesions of bone: Evaluation by FDG PET. 41: 1695-1701
(2) Clin Nucl Med 2000; Franzius C, et al. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. 25: 874-881
Thyroid Cancer:
General:
I-131 scanning has been the mainstay for the evaluation of recurrent thyroid carcinoma. Patients who are found to have I-131 accumulating local recurrences or metastases on diagnostic scans will usually receive subsequent therapeutic I-131 doses which may be curative. Unfortunately, not all recurrences are iodine avid (50-60% of papillary and 64-67% of follicular cancer recurrences are iodine avid [4]). Patients with elevated human thyroglobulin levels, but negative I-131 scans pose a diagnostic and therapeutic dilemma. Although therapy with I-131 has been performed in these patients with subsequent decline in thyroglobulin levels [5], one can never be certain if all sites of disease have been adequately treated. Surgical resection of untreated sites of disease would certainly be desirable [4]. Other agents such as Tc-sestamibi, Tc-tetrofosmin, and thallium-201 have all be used to evaluate for non-iodine avid recurrent cancer and metastatic disease. Although useful for locoregional disease, physiologic accumulation of these agents in other organs (particularly within the abdomen) limits their usefulness for whole body examinations. FDG PET imaging has also shown great promise in the detection of metastatic disease in post-thyroidectomy/I-131 ablation patients [1,2]. Unlike conventional imaging, FDG PET imaging can detect metastatic disease to normal sized lymph nodes [1]. I-131 scintigraphy and FDG PET imaging are complementary diagnostic methods for the evaluation of recurrent or metastatic thyroid cancer [3,6]. The iodine uptake of a tumor is inversely related to its glucose metabolism [3]. Tumor uptake is also related to the degree of tumor differentiation [4]. In general, highly differentiated thyroid carcinoma will be I-131 positive and FDG PET negative, while less differentiated cancers will show the reverse (i.e.: FDG PET positive and I-131 negative) [1,6]. It is important to remember that although FDG-positive recurrences may indicate dedifferentiated and more aggressive tumors, this finding does not automatically predict a worse prognosis [4]. Interestingly, patients with higher thyroglobulin levels (above 100 ug/L) have been shown to have a higher incidence of true-positive FDG PET exams [4]. Another benefit of FDG PET imaging is the identification of unsuspected secondary malignancies [4].
One of the benefits of FDG PET imaging is that patients do not need to discontinue hormonal replacement therapy prior to imaging, however, one article has suggested that lesion detection is improved when performing the exam during TSH stimulation (following exogenous thyroid hormone removal) [3]. Further studies will be required to determine the optimal method for the FDG PET examination in thyroid cancer patients.
FDG PET Imaging in Patients with Suspected Recurrent Thyroid Cancer and Negative I-131 Scans:
For patients with negative I-131 scans, FDG PET imaging has a reported sensitivity of 69-94%, and specificity of 42-95% [1,4]. Despite this variability, FDG PET imaging can have significant impact on patient management in this difficult subset of patients. Treatment can be changed in over 50% of patients based upon FDG PET imaging results [4]. FDG PET imaging should be viewed as another tool for assisting in the detection of non-iodine avid thyroid metastases.
Non-pathologic findings:
Following thyroidectomy, FDG uptake in the thyroid bed is a common finding and does not necessarily indicate recurrent disease unless a definite mass can be identified with other imaging modalities [2]. False positive cases have occurred in association with mediastinal tuberculous adenitis.
REFERENCES:
(1) J Nucl Med 1999; Chung JK, et al. Value of FDG PET in papillary thyroid carcinoma with negative I-131 whole body scan. 40: 986-992
(2) J Nucl Med 2000; Alnafisi NS, et al. FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. 41: 1010-1015
(3) J Nucl Med 2000; Moog F, et al. Influence of thyroid-stimulating hormone levels on uptake of FDG in recurrent and metastatic differentiated thyroid carcinoma. 41: 1989-1995
(4) J Nucl Med 2001; Schluter B, et al. Impact of FDG PET on patientw with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131-I scan. 42: 71-76
(5) Thyroid 1994; Clark OH, et al. Managament of patients with differentiated thyroid cancer who have positive serum thyroglobulin levels and negative radioiodine scans. 4 (4): 501-505
(6) J Nucl Med 2001; Shiga T, et al. Comparison of 18F-FDG, 131I-Na, and 201-Tl in diagnosis of recurrent or metastatic thyroid cancer. 42: 414-419