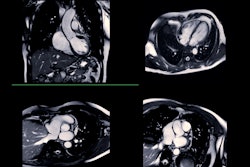
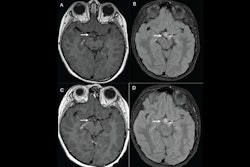
Guerbet has received approval from the U.S. Food and Drug Administration (FDA) to expand the label for its Elucirem (gadopiclenol) gadolinium-based contrast agent to include pediatric patients ages 2 and younger, including term neonates.
Elucirem is a macrocyclic high-relaxivity gadolinium-based contrast agent (GBCA) approved for use with MRI to detect and visualize lesions with abnormal vascularity in the central nervous system and the body, including the head and neck, thorax, abdomen, pelvis, and musculoskeletal system, according to Guerbet. It is administered at half the conventional gadolinium dose compared to existing nonspecific contrast agents, the company added.
The FDA originally approved Elucirem for adults in September 2022, and the European Medicines Agency granted approval in December 2023, the firm noted.