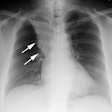
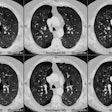
Radiation Pneumonitis:
View cases of radiation pneumonitis
Clinical:
Advanced radiotherapy has been developed to conform the
radiation dose to the tumor and spare adjacent normal tissues
[11]. These include 3D-CRT, IMRT, and SBRT [11]. 3D-CRT involves
use of volumetric CT to conform multiple radiation beams to the
tumor geometry [11]. Intensity-modulated RT (IMRT) is superior
to 3D-CRT because structures are "weighted" according to their
importance, and then an optimal plan is devised by using
internal planning system algorithms [11]. Additionally, dynamic
multileaf collimators are incorporated, which allows
modifications of the dose intensity within the treatment field
improving the ability to conform to the target tumor [11].
Sterotatic body RT (SBRT) involves localized alative radiation
delivered to regions at multiple body sites over a smaller
number of fractions (higher radiation dose per fraction) with
tighter margins [11]. Finally, proton beam therapy involves use
of protons (charged particles) which have a lower entrance
radiation dose and the peak dose is delivered with the Bragg
peak at the tumor and a minimal exit dose [11]. The use of
proton therapy has been shown to significantly reduce the dose
delivered to normal tissues [11].
Therapeutic doses of radiation cause virtually all patients to have some degree of lung parenchymal reaction, however, the findings are not always radiographically apparent [3]. The factors which affect the likelihood for lung injury include the volume of irradiated lung, the total dose of radiation delivered, and and the fractionation of the dose [6]. The incidence of clinical symptoms related to radiation penumonitis correlate with the mean lung dose and the percentage of lung volume that receive a radiation dose of at least 20 Gy [11]. Radiographic findings of radiation pneumonitis are not usually seen at doses below 2000 to 3000 rads (20-30 Gy) [6,11]. Clinically significant radiation pneumonitis develops in 50% of patients with fractionated doses exceeding 3500-4000 rads, and is nearly always present above 4000 rads (40 Gy) [6,11]. The affected lung usually conforms to the radiation port, although occasionally the acute changes of radiation pneumonitis can be seen outside the margins of the radiation field [3]. Three-dimensional conformal radiation therapy has been developed to minimize irradiation of surrounding normal structures [9].
The severity of radiation pneumonitis
increases with increased volume of tissue radiated, increased
radiation dose, and a faster rate of administration [3]. A
single dose of 750 rads delivered
to both lungs may produce a severe or fatal radiation injury.
Chemotherapeutic agents such as actinomycin
D, adriamycin, bleomycin,and
busulfan can potentiate the effects
of radiation [6]. Other factors that increase the risk for toxic
lung effects include older age, smoking history, and pulmonary
hypertension [11].
Treatment is most commonly high dose corticosteroids [11].
Irradiation of the heart can lead to coronary artery and
valvular heart disease [11]. Factors associated with an
increased risk include a cardiac dose of >30 Gy, high
radiation per fraction (> 2 Gy/day), younger age (< 50
years), and concomitant chemotherapy, especially anthracyclines
[11].
Another long term complication of radiation therapy to the
lungs is an increased risk for lung cancer [9]. The risk
increases with time after treatment with the median time
interval being approximately 9.6 years [9].
Osteoradionecrosis, also known as radiation osteitis, is
another late complication of radiation therapy that results from
impaired bone circulation [11]. It occurs one or more years
after XRT, but is found in only 0.3-1.8% of patients [11].
Osteoradionecrosis results in bone atrophy and, in turn,
attempts to repair that lead to the deposition of new bone and
ischemic trabeculae [11]. Radiographs and CT will demonstrate
heterogeneous bone density with alternating areas of osteopenia,
increased density, coarse trabeculation, and mottling [11].
There are 2 forms of radiation lung injury: early phase radiation pneumonitis and later, chronic radiation fibrosis [9].
1- Acute (Early): Exudative
phase:
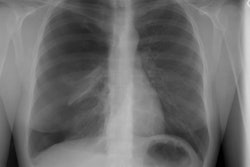
Acute radiation pneumonitis is characterized by edema (due to increased capillary permeability), diffuse alveolar damage, desquamation of the capillary endothelium, and the development of hyaline membranes. There is regional vasoconstriction secondary to alveolar hypoxia. The lung may recover completely, but more commonly there is progression to a proliferative phase that eventually leads to fibrosis as the process resolves. There is usually a latent period between radiation exposure and the development of acute radiation pneumonitis- typically 1 week to 7 months following treatment (peak 4-12 weeks). An increased risk for acute radiation injury is seen in patients that receive concurrent chemotherapy. A second course of radiotherapy is also more likely to induce significant pneumonitis [3]. A large percentage of patients may be asymptomatic. In fact, only about 8% of patients will develop symptomatic pneumonitis [2]. Symptoms include dyspnea (in up to 93% of cases which can be mild to severe) and a generally non-productive cough (up to 58%). Patients may have a low grade transient fever (7%). Hemoptysis is rare. Treatment is with corticosteroids. Steroid prophylaxis has not been shown to be of benefit in patients receiving XRT to the lungs [2].
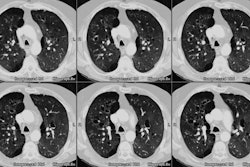
Initially, CXR reveals hazy consolidation in the affected
radiated areas approximately 3-4 weeks following treatment
(sometimes areas outside the portal can also be affected [3,6]). Next, these areas become more
consolidated and can contain air bronchograms.
The areas of consolidation typically have relatively sharp
margins which do not follow the pattern of lung anatomy, but
rather the shape and size of the radiation ports.
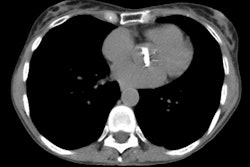
CT is more sensitive than conventional radiography in the detection of radiation induced parenchymal lung injury [3]. On CT, there are areas of ground-glass attenuation or consolidation, or both, which conform closely to the radiation ports. Some overly septal thickening producing a "crazy-paving" pattern can develop [11]. A nodule-like pattern of radiation pneumonitis can also sometimes be seen [11]. Findings may be seen outside the radiation field in up to 20% of cases [3]. Pleural effusions can uncommonly occur- usually within 6 months of treatment or in association with acute radiation pneumonitis [3]. The effusion will usually spontaneously resolve [3]. A rapid increase in the size of the effusion or reaccumulation after thoracentesis suggests a malignant effusion [3]. Effusions which develop after 6 months of completion of radiation therapy should also be evaluated with thoracentesis to exclude malignant disease [8]. Pericardial effusions may also occur- typically within 6-9 months of completion of therapy [8].
On scintigraphic imaging the findings can be similar to PE with preserved ventilation and absent perfusion, but the defect generally does not conform to segmental boundaries, rather it conforms to the radiation port.
2- Chronic (Late): Fibrotic Phase or Radiation
Fibrosis
Chronic or fibrotic changes are almost exclusively seen within the margins of the radiation field and can occur even in patients with no previous evidence of clinical acute radiation pneumonitis [3]. Chronic radiation fibrosis develops 3 to 12 months after radiation therapy ends [8], but can continue to evolve for up to 2 years following SBRT [10], and is characterized by permanent damage to the endothelium and Type I pneumoctyes. There is irreversible decreased pulmonary perfusion due to vessel obliteration. Affected patients can be asymptomatic, or have varying degrees of dyspnea. The CXR reveals sharply a marginated area of fibrosis, volume loss, and pleural thickening which conform to the treatment portals, rather than anatomic boundaries [6]. On CT there are streaky opacities, volume loss, pleural thickening, solid consolidation (doses over 5000 cGy), and traction bronchiectasis within the regions subtended by the radiation ports. The abnormalities usually remain stable after 2 years [3,4]. More complex 3D conformational radiation therapy generally produces consolidation, volume loss, and bronchiectasis similar to, but less severe than conventional radiation therapy. However, it can also produce more complex appearing radiographic abnormalities including a mass-like pattern [6].
Findings which suggest recurrent tumor in an area of radiation fibrosis include alterations in the straight, smooth contour (developing convex bulge), an enlarging consolidation within a region of established fibrosis, and soft tissue filling in of dilated bronchi [6,7,8]. FDG PET imaging can confirm the presence of recurrent tumor, but imaging is best delayed for at least 3 months (others say 6 months [11]) following therapy to decrease false-positive findings associated with tracer uptake in areas of inflammation [6,9]. However, persistent uptake in areas of radiation pneumonitis can be seen for up to 15 months after completion of XRT [9].
REFERENCES:
(1) Radiol Clin North Am 1991; Moore EH. Diffuse lung disease in the current spectrum of immunocompromised hosts (non-AIDS). 29(5): 983-997
(2) Chest 1997; Pulmonary radiation injury. 111: 1061-76 (No abstract available)
(3) AJR 1998; Logan PM. Thoracic manifestations of external beam radiotherapy. 171: 569-577 (Review. No abstract available.)
(4) Radiology 1999; Libshitz HI, et al. Filling in of radiation therapy-induced bronchiectatic change: A reliable sign of locally recurrent lung cancer. 210: 25-27
(5) Radiology 2001; Rosen II, et al. Correlation between lung fibrosis and radiation therapy dose after concurrent radiation therapy and chemotherapy for limited small cell lung cancer. 221: 614-622
(6) Radiographics 2004; Choi YW, et al. Effects of radiation therapy on the lung: radiologic appearances and differential diagnosis. 24: 985-998
(7) Radiol Clin N Am 2005Munden RF, Bruzzi J. Imaging non-small cell lung cancer. 43: 467-480
(8) Radiology 2005; Munden RF, et al. Imaging of the patient with non-small cell lung cancer. 237: 803-818
(9) Radiographics 2011; Larici AR, et al. Lung abnormalities at
multimodality imaging after radiation therapy for non-small cell
lung cancer. 31: 771-789
(10) Radiographics 2018; Febbo JA, et al. Sterotactic body
radiation therapy for early-stage non-small cell lung cancer: a
primer for radiologists. 38: 1312-1336
(11) Radiographics 2019; Benveniste MF, et al. Recognizing
radiation therapy-related complications in the chest. 39:
344-366