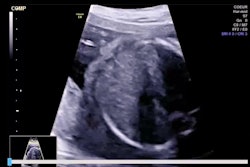
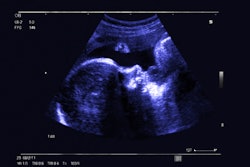
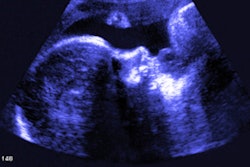
Israeli firm Pulsenmore has secured de novo clearance from the U.S. Food and Drug Administration (FDA) for its home-use prenatal ultrasound device, Pulsenmore ES.
The device allows pregnant women to perform ultrasound scans at home and is connected to the user's smartphone. Exam directions are provided via an app, and are interpreted remotely by physician readers, the company said. It is already in use in Israel, Europe, Brazil, and Australia.
"Pulsenmore ES does not replace in-clinic diagnostic or anatomical ultrasound examinations, but complements existing workflows in alignment with the American College of Obstetrics and Gynecology Guidance for Tailored Prenatal Care Delivery for Pregnant Individuals," Pulsenmore noted.
The firm is preparing for a phased U.S. launch in early 2026.