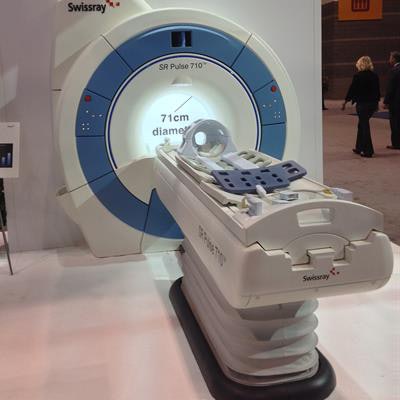
Swissray International is embarking on a new path in 2017. The longtime player in the digital radiography (DR) segment is moving into MRI with its first scanner, a wide-bore system called SR Pulse 710 that is set to begin shipping later this year.
SR Pulse 710 is a 1.5-tesla scanner with a 71-cm bore that has received clearance from the U.S. Food and Drug Administration (FDA). Swissray sees the product as a way to diversify from an increasingly competitive DR market.
 Gilbert Wai, Swissray's CEO.
Gilbert Wai, Swissray's CEO."DR is where we built our company name," said Gilbert Wai, Swissray's CEO. "The DR market has become more competitive right now. We should step out from DR and into other imaging modalities, and that is the direction we are taking."
DR foundation
Digital radiography has been Swissray's prime product since 1997, when the company received FDA clearance for its first DR device. Since then, as more companies introduced their versions of DR devices, competition has naturally intensified.
Three years ago this month, Swissray began to diversify its product line with the acquisition of Norland's bone densitometry assets from Cooper Surgical. Because bone densitometry relies on x-ray, the move was not a quantum leap from Swissray's DR roots.
While DR has served Swissray well over the past two decades, one key reason for the move into MRI is that developed countries have become "well-saturated" with DR systems, now focusing more on replacement units rather than first-time purchases, Wai said.
"In terms of new [DR] expansion, it has to be in emerging markets. I would cite China, India, Asia, and Indonesia," he added. "Some of the big countries in Africa are on the surface of installing computed radiography and then DR."
Wai expects the worldwide DR market to grow by single-digit percentages in the near future, which makes a compelling argument for the company's strategy to diversify its product line.
MRI partnership
Swissray is partnering with Alltech Medical Systems, based in Chengdu, the capital of Sichuan province in China. SR Pulse 710 is manufactured at the company's U.S. affiliate, Alltech Medical Systems America in Cleveland.
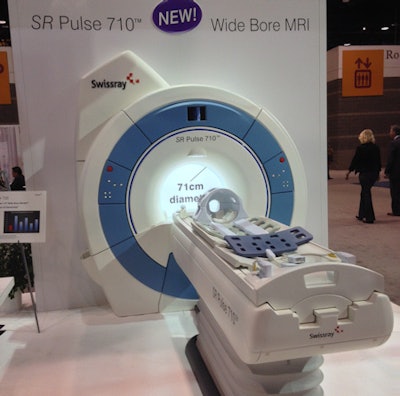 Swissay debuted its SR Pulse 710 MRI at RSNA 2016.
Swissay debuted its SR Pulse 710 MRI at RSNA 2016.Alltech currently manufactures a 60-cm-bore MRI scanner, which the company markets as Echostar in Asia and Europe. The 71-cm bore SR Pulse 710 comes from that platform.
"With our bore of 71 cm, every centimeter counts with large patients," said Anne Sheehan, Swissray's director of MRI. "It's not just about claustrophobia. In the U.S., there is a lot of obesity, and you need to image everyone who walks in the door."
Swissray has been beta-testing its SR Pulse 710 since March of last year through Advantage Diagnostics, which operates six outpatient imaging centers in Ohio. One facility has been scanning an average of 15 patients per day with the system, and a second installation at another location is planned for the first half of this year.
"We think it will be quite the workhorse for diagnostic imaging centers, small and medium hospitals, and orthopedic clinics," Sheehan added.
Beyond its wide bore, the system offers a gradient strength of 33 millitesla per meter, a 50 x 50 x 50-cm field-of-view, and a 16-channel radiofrequency system. The table can handle patients weighing as much as 550 lb, and a variety of dedicated orthopedic surface coils are available for the knee, shoulder, and wrist to aid in long-bone and whole-body imaging. The scanner also features a zero boil-off magnet designed for a helium refill every 10 years.
Bottom-line economics
Swissray also plans to tout the scanner's price point compared with comparable wide-bore systems, along with a lower total cost of ownership and subsequent quicker return on investment, Sheehan said.
"Wide-bore magnets are generally selling above the $1 million mark, and there are very few refurbished systems available below that [price]," she said. "We hope to come in below that."
Swissray's entrance into the U.S. MRI market may come at a most opportune time. The average age of the installed base of MRI scanners in the U.S. advanced from 8.7 years in 2010 to 11.4 years in 2013, according to a February 2014 research report by IMV Medical Information Division.
Given that the last spark of new MRI installations took place from 2002 to 2004, the U.S. market may be ready to replace older units. In fact, 20% of the 450 MRI administrators surveyed by IMV said they plan on purchasing a new system in the next three years, which brings the replacement timetable to 2017.