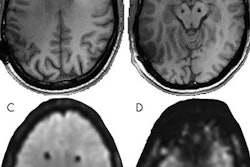
Interventional device developer Medtronic announced that the U.S. Food and Drug Administration (FDA) has cleared parts of its Activa portfolio of deep brain stimulation neurostimulators for full-body MRI scans.
The clearance expands patient access to care, making it safe to undergo MR exams while also undergoing deep brain stimulation therapy.
Medtronic's MR-conditional deep brain stimulation systems are the only ones that give patients access to full-body MRI scans, the company said.