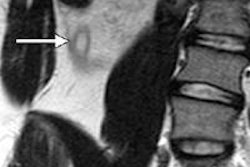
MRI equipment manufacturer ClearMRI Solutions has received U.S. Food and Drug Administration (FDA) clearance for its new Helios-RG8 digital broadband MRI coil accessory kit.
The clearance allows imaging facilities with 1.5-tesla GE Healthcare four-channel 9.x legacy MRI scanners to upgrade to eight-channel coils, ClearMRI said.
The kit extends the profitable life of older MRI systems by upgrading them to eight-channel architecture, and it enables centers to improve their images without replacing the MRI scanner, the company said.