Researchers have developed a PET radiotracer that reveals activity in brain cells that may be involved in the early stages of Alzheimer's disease, according to a study published January 27 in the Journal of Nuclear Medicine.
An international team led by Drs. Christopher Rowe of Austin Health in Melbourne, Australia, and Nobuyuki Okamura, PhD, of Tohoku Medical and Pharmaceutical University in Sendai, Japan, tested the performance of a tracer named F-18 SMBT-1 for revealing the activity of reactive astrocytes, which is believed to be an early trigger for beta amyloid deposits and cognitive decline. They found increased levels of F-18 SMBT-1 in patients who were in the preclinical stages of beta amyloid accumulation.
"F-18 SMBT-1 will allow a better understanding of the pathophysiology of [Alzheimer's disease], enabling more accurate staging and prognosis at earlier stages of the disease," the group wrote.
Astrocytes are the most abundant cells in the brain and are involved in a host of normal activity, but research has shown that they can become reactive and trigger inflammation that leads to amyloid plaque accumulation, a well-established sign of Alzheimer's disease.
Reactive astrocytes overproduce monoamine oxidase-B (MAO-B), an enzyme on the outer membrane of the cells. The F-18 SMBT-1 tracer was designed to bind to MAO-B and emit signals picked up on PET scans. This allows researchers to visualize the early involvement of this cellular activity in neurodegeneration.
In a study also published January 27 in the Journal of Nuclear Medicine, the group confirmed for the first time in humans that F-18 SMBT-1 is a highly selective MAO-B tracer. They showed the pattern of the tracer throughout the brain in patients matched the spread of MAO-B in the brain seen in Alzheimer's disease lab studies.
In this study, the group investigated the performance of F-18 SMBT-1 PET across the continuum of Alzheimer's disease, from groups of patients with beta-amyloid deposits who were cognitively normal to patients in later stages of the disease.
The group enrolled a total of 77 nonsmoking elderly volunteers between the ages of 58 and 89 from a community in Melbourne, Australia. Fifty-seven participants were cognitively unimpaired, 12 had mild cognitive impairment, and eight patients had Alzheimer's disease. All 77 patients had standard amyloid PET scans to establish positive or negative baseline amyloid levels, followed by F-18 SMBT-1 PET scans (Gemini TF 64 PET/CT, Philips).
The researchers found no significant differences in F-18 SMBT-1 levels in beta amyloid-negative patients with mild cognitive impairment or who were cognitively normal. Conversely, F-18 SMBT-1 binding to MAO-B on reactive astrocytes was significantly higher in beta amyloid-positive Alzheimer's disease patients, according to the findings.
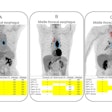
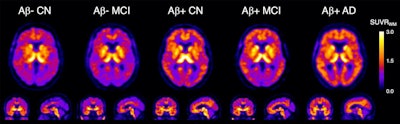 Representative transaxial, coronal, and sagittal F-18 SMBT-1 PET images of study participants: (from left) beta amyloid-negative cognitively normal (CN), beta amyloid-negative mild cognitively impaired (MCI), beta amyloid-positive CN, beta amyloid-positive MCI, and beta amyloid-positive with Alzheimer's disease. In beta amyloid-negative CN and MCI, the normal distribution of F-18 SMBT-1 in the brain is observed, highlighting cortical areas with high concentration of MAO-B, such as the basal ganglia, thalamus, mesial temporal cortex, and anterior cingulate, as well as the different nuclei in the brainstem. Higher cortical F-18 SMBT-1 binding is observed in beta amyloid-positive CN, MCI and Alzheimer's disease participants, with binding extending to frontal, temporal, occipital, and posterior cingulate. Image courtesy of the Journal of Nuclear Medicine.
Representative transaxial, coronal, and sagittal F-18 SMBT-1 PET images of study participants: (from left) beta amyloid-negative cognitively normal (CN), beta amyloid-negative mild cognitively impaired (MCI), beta amyloid-positive CN, beta amyloid-positive MCI, and beta amyloid-positive with Alzheimer's disease. In beta amyloid-negative CN and MCI, the normal distribution of F-18 SMBT-1 in the brain is observed, highlighting cortical areas with high concentration of MAO-B, such as the basal ganglia, thalamus, mesial temporal cortex, and anterior cingulate, as well as the different nuclei in the brainstem. Higher cortical F-18 SMBT-1 binding is observed in beta amyloid-positive CN, MCI and Alzheimer's disease participants, with binding extending to frontal, temporal, occipital, and posterior cingulate. Image courtesy of the Journal of Nuclear Medicine.Most importantly, F-18 SMBT-1 binding was significantly higher in the same brain regions in beta amyloid-positive and cognitively normal patients compared to beta amyloid-negative and cognitively normal patients, the researchers stated. When all clinical groups were considered together, F-18 SMBT-1 was highly correlated with beta-amyloid burden, they concluded.
"These findings suggest that increased F-18 SMBT-1 binding is detectable at the preclinical stages of [beta amyloid] accumulation, providing strong support for its use as surrogate marker of astrogliosis in the [Alzheimer's disease] continuum," the authors wrote.
The researchers wrote that to the best of their knowledge, F-18 SMBT-1 is the first available F-18-labeled MAO-B tracer to be used in a clinical study to assess reactive astrogliosis. This process plays a key early role in Alzheimer's disease, as well as in several other neurodegenerative conditions, from neuropsychiatric conditions like depression to movement disorders, they added.
"In the particular case of assessing astrogliosis, F-18 SMBT-1 will allow a better understanding of the pathophysiology of [Alzheimer's disease], while examining its potential direct or indirect effect over neurodegeneration," they wrote.