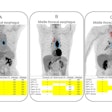
Amyloid PET scans are associated with changes in the care plans in ethnoracially diverse, clinically heterogeneous populations, researchers have reported.
The finding is significant, as Black, Latinx, and other races have not been adequately represented in prior studies, noted lead author Charles Windon, MD, of the University of California, San Francisco, and colleagues.
"This finding emphasizes that the utility of amyloid PET in clinical decision making is applicable for all individuals, regardless of ethnoracial identity," the group wrote. The work used data from the New IDEAS study, which began in 2020; Windon and colleagues' analysis was published July 29 in Alzheimer’s and Dementia.
Black older adults are twice as likely, and Latinx older adults 1.5 times as likely, to have clinical Alzheimer’s disease and related dementias compared to white older adults, the authors explained. However, Alzheimer’s disease biomarker research is notable for limited inclusion of ethnoracially diverse individuals, they wrote.
For instance, research called the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study concluded in 2019 that amyloid PET was associated with a more than 60% change in clinical management (between pre-PET and 90 days post-PET) among Medicare beneficiaries with mild cognitive impairment or dementia. The study included more than 15,000 individuals, but less than 10% of the sample consisted of non-white individuals
The American College of Radiology (ACR) and the Alzheimer's Association launched the New IDEAS study in 2020. It ran through December 2024 with the goal of assessing changes in clinical management among ethnoracially diverse, clinically heterogeneous patients with mild cognitive impairment.
According to this current analysis of the IDEAS data, among 5,757 participants (median age 75 years; 21.7% Black, 20.3% Latinx, and 58.1% all other races/ethnicities), changes in care plans occurred in 59% of individuals 90 days after amyloid PET scans. The most frequent changes, across all ethnoracial and clinical presentation subgroups, were related to Alzheimer’s disease medication use, followed by modifications to non-Alzheimer's disease medications, and finally changes to counseling.
In addition, changes in overall diagnosis (Alzheimer's disease to non-Alzheimer's disease or non-Alzheimer's disease to Alzheimer's disease) occurred for 32.4% of Black participants, 29.4% of Latinx participants, and 29.5% of other races/ethnicities participants, the researchers reported.
"Overall, we replicated findings from the original IDEAS study within a more inclusive cohort, extending the generalizability of the results and further validating the benefits of amyloid PET in a real-world care setting," the group wrote.
Notably, the researchers added that the amyloid-targeting monoclonal antibody lecanemab (Leqembi, Eisai and Biogen) received traditional FDA approval in July 2023, which allowed them to capture early data on the use of the novel medication toward the latter part of the study’s enrollment window. Based on PET scan results, clinicians in the study made changes in the care plan and recommended the use of lecanemab to more than 10% of patients.
"We believe these data represent some of the earliest real-world prescriber data for lecanemab," the group wrote.
Ultimately, based on the results of the New IDEAS study, increasing access to amyloid PET has significant implications for management outcomes among ethnoracially diverse populations, the researchers concluded.
The full study is available here.