Claritas HealthTech subsidiary Claritas NucMed Technologies has received clearance from the U.S. Food and Drug Administration (FDA) for its latest PET software.
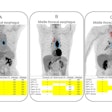
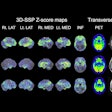
Called iPETcertum, the software enhances PET images and automates segmentation and quantification. Using the software, clinicians can click on regions of interest (tumors/lesions) in PET, PET/CT, or PET/MRI scans and quickly assess standardized uptake values (SUV) and volumes that otherwise could take several hours manually, Claritas NucMed said. The display of segmentation regions is based on clinician-defined parameters (minimum/maximum thresholds) of SUV and volume values, the company added.
In addition, the software’s image enhancement capability removes noise and thereby improves visibility in scans. Noisy scans obtained with reduced isotope dose and/or reduced scan times can be enhanced to yield reliable diagnostic quality images, the company said.
IPETcertum is vendor-neutral and works with all types of radionuclides, Claritas NucMed noted.