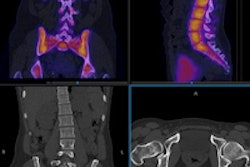
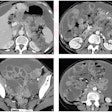
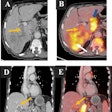
The U.S. Food and Drug Administration (FDA) has cleared Orpheus Medical's new clinical video and imaging documentation platform, which also includes a mobile application.
The company's system provides video capture, archive, and live-stream capabilities from any department and any video source, Orpheus Medical said. It integrates with a facility's electronic medical record as well as its PACS. Its mobile application allows clinicians to capture medical video, images, and audio files from their mobile devices.
The company plans to showcase its platform at this week's Healthcare Information and Management Systems Society (HIMSS) meeting in Las Vegas.