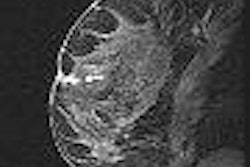
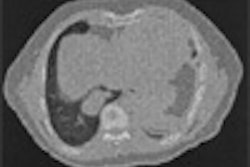
Israeli focused-ultrasound treatment developer InSightec reported that it has completed treatment of the final patient in a postmarketing study to evaluate the efficacy of its ExAblate 2000 in treating uterine fibroids in African-American women.
The postmarketing study is an element of the FDA's commercial approval for ExAblate 2000 in October 2004 for use in patients with uterine fibroids, the Haifa-based firm said.
The device utilizes MR-guided focused ultrasound (MRgFUS) to thermally ablate tissue.
The study was initiated to confirm the efficacy of the ExAblate 2000 treatment in African-American women, as they tend to have more severe uterine fibroids, the company said. The product is being used at 30 sites worldwide and has treated more than 1,500 women with uterine fibroids, according to InSightec.
By AuntMinnie.com staff writers
March 21, 2006
Related Reading
MRgFUS provides effective, noninvasive treatment for uterine fibroids, November 30, 2005
InSightec partners with ACRIN, November 22, 2005
InSightec gets Texas install, October 11, 2005
InSightec begins clinical trial enrollment, September 15, 2005
GE, Insightec ink new deal, August 17, 2005
Copyright © 2006 AuntMinnie.com