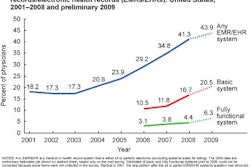
"Meaningful use" has become the holy grail of healthcare IT -- the standard that providers must achieve to receive stimulus funds. U.S. regulators recently issued final rules that define how they view meaningful use, but the guidelines raise almost as many questions as they answer.
Issued in July by the Office of the National Coordinator for Health Information Technology (ONC), the final rule covers certification of electronic health record (EHR) products to be used to achieve the Centers for Medicare and Medicaid Services (CMS) meaningful use requirements. But questions have been flying fast and furious with respect to how certification will be achieved in the real world.
CMS has said it will open the registration process to apply for meaningful use funding in January 2011. The testing and certification of complete EHR systems by this date may be achievable, due to the limited number of soup-to-nuts EHR systems commercially available. However, there are hundreds of healthcare IT products that qualify as EHR modules, including RIS and PACS applications. These also perform "meaningful use" functions for the healthcare providers who've already gone digital and utilize electronic health management technology. All need to be tested.
The ONC has named three authorized testing and certification bodies (ONC-ATCBs) to handle applicants: InfoGuard Laboratories of San Luis Obispo, CA; the Certification Commission for Health Information Technology (CCHIT) of Chicago; and the Drummond Group of Austin, TX.
Twenty questions
To try to resolve the many questions regarding how certification will be implemented, on September 22 the ONC published answers to 20 of the most frequently asked questions it has received. Some of these may be real eye-openers for healthcare organization or physician practices that aren't already using EHR technology
For example, certification of an EHR module will guarantee that it can perform the certification function or functions for which it was tested. (By ONC definition, an EHR module can bundle up to nine clinical quality measures.)
But certification does not guarantee compatibility with any other certified EHR module. ONC spokesperson Nancy Szemraj explained the rationale for not testing for interoperability.
"The ONC-ATCBs test and certify to the criteria adopted by the standards and certification rules, which are intended to assure the capability of users to meet the phase I meaningful use criteria. Interconnectivity is addressed at this point via the exchange requirements," she wrote in an e-mail to AuntMinnie.com.
"The use of EHR modules is voluntary and available for those healthcare providers who seek to implement a customized EHR technology solution that can best meet their needs," she explained. "Eligible healthcare providers that seek to implement a customized solution with multiple EHR modules also bear the responsibility to ensure that those EHR modules work together."
From a practical standpoint, nothing has changed for hospitals and physician practices already using best-of-breed systems. Resolving interoperability problems has always been their headache.
But the ONC's rules will not make the conversion to digital easier for the uninitiated with limited budgets and limited IT personnel resources, who may presume the ONC-certified stamp of approval equates to plug-and-play. It may also give a market advantage to vendors who sell a complete EHR solution. Time will tell.
Meaningful use for radiology
For imaging centers and radiology practices that are or will be able to meet meaningful use criteria by January 2011, the situation may be more onerous. AuntMinnie.com asked whether the certified health IT products list that will be posted on the ONC website will include a section for RIS and PACS products by vendor product and software version.
"The first stage of meaningful use and certification is focusing on EHRs," Szemraj responded. "The modules you identify are not part of the first stage of meaningful use requirements and, therefore, would not be expected to be on the certified health IT products list for phase I meaningful use criteria."
However, Bill Smith, the chief financial officer of the Drummond Group, told AuntMinnie.com that as long as products like RIS and PACS include EHR modules, the company was prepared to test the modules. Applications are being processed in the order received.
Other concerns were clarified in ONC's new frequently asked questions posting.
IT systems that act as data sources, such as a registration system, and are not intended to perform required capabilities do not need to be certified simply because they supply data to a complete EHR or EHR module.
In what may be a nod to faster implementation of computerized physician order-entry (CPOE) software for diagnostic imaging, all of the capabilities specified in the ONC standards must be tested and certified at the same time. Vendors or self-developers of CPOE systems cannot elect to have only the prescription-ordering capability tested. Purchasers of certified CPOE systems will have the ability to use them to order lab and radiology tests, even though this is not a requirement of phase I meaningful use.
The ONC reiterated that self-developed, noncommercial healthcare IT software can be submitted for certification as either a complete system or as individual or bundled modules. If a vendor decides not to certify a particular software version of its product, a healthcare provider independently may do so, as long as this action does not violate any vendor-customer contractual agreement.
All costs for testing and certification in these situations will be paid by the healthcare provider. Unaffiliated healthcare providers may jointly submit an application and jointly pay for testing. As an example of the cost to do this, the Drummond Group has quoted a rate of $625 per module for up to eight modules, $11,500 for a group of 10 to 19 modules, and $16,000 for 20 or more modules per application.
Both commercial and self-developed software may be combined to create a complete EHR system, as long as all components that comprise the system are certified.
Vendors who have either a complete EHR system or an EHR module containing multiple integrated modules tested and certified cannot sell any of these components individually as certified modules unless they are individually tested and certified. Similarly, if a complete EHR system or a multiple integrated module EHR fails testing, an applicant may request that individual components be tested. The ONC advised that "whether an ONC-ATCB would choose to honor a request for a change, as well as any costs associated with a change, would depend upon the arrangement between the applicant and the ATCB."
When an EHR module containing multiple integrated submodule capabilities is submitted for privacy and security certification, this will be done as a whole with a single cost. Submodules will not be individually tested for the privacy and security certification when submitted in a single bundled EHR module.
Certified modules or complete EHR systems that are sold under multiple brand names do not have to be recertified for each brand name as long as requirements are met by the resellers. As long as ONC receives proper notification, all brands of the OEM product(s) will be listed on the ONC website.
If two or more IT products are used to create a single certification requirement, it is necessary for all products to be tested to be compliant.
A data warehouse must be certified if it is used to submit calculated clinical quality measures.
Interface engines per se do not require certification. They must be certified if they provide a meaningful use function, such as the capability to record, modify, retrieve, and submit information in a standardized format.
In situations where a healthcare provider has purchased software from different vendors that has overlapping capabilities and only uses one, or in situations where a healthcare provider uses multiple products that perform the same function for different users within the organization, as long as all products are certified, this is acceptable. The healthcare provider will be responsible for reconciling the data between the multiple products for purposes of reporting the data to federal and state agencies.
If a certified software EHR module needs to be reconfigured to work with another software program, this is allowable without compromising its certified status as long as its performance as an EHR module is not adversely affected.
ONC's complete set of questions and answers may be accessed by clicking here. The U.S. National Institute of Standards and Technology's (NIST) 45 approved test procedures can be accessed here.
By Cynthia E. Keen
AuntMinnie.com staff writer
September 30, 2010
Related Reading
ONC announces certification firms, August 31, 2010
ONC webinar to address EHR certification, August 24, 2010
Meaningful use: Comply and get paid ... but how? August 23, 2010
Final meaningful use rules: What radiologists need to know, July 29, 2010
Copyright © 2010 AuntMinnie.com