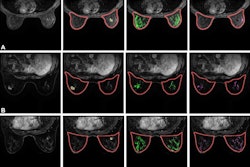
Chicago-based AI developer SimBioSys has received clearance from the U.S. Food and Drug Administration (FDA) for the latest version of its MRI breast cancer platform TumorSight Viz.
TumorSight Viz 1.3 builds on the platform's foundation with new capabilities that significantly improve performance, including improved AI-driven segmentation, faster case processing, and new PACS connectivity that automates image transfer and reduces manual tasks, the company said.
The platform uses dynamic contrast-enhanced MRI data to build patient-specific 3D digital models of breast cancer tumors and was initially cleared by the FDA in 2024.