Dynamic contrast-enhanced MRI (DCE-MRI) performs well in predicting early response to immunochemotherapy in women with triple-negative breast cancer, according to a study presented December 6 at the San Antonio Breast Cancer Symposium.
Tumor volume reduction shown on DCE-MRI early into neoadjuvant immunochemotherapy regimens can predict treatment response in these women, said Gaiane Rauch from the University of Texas MD Anderson Cancer Center in Houston in her presentation.
“Higher accuracy of prediction was seen after four cycles of treatment than after two cycles of neoadjuvant immunochemotherapy,” Rauch said.
There remains a need for noninvasive biomarkers for response prediction of neoadjuvant immunochemotherapy in triple-negative breast cancer. Rauch said such markers can guide the least toxic and most effective treatment regimens.
Neoadjuvant immunotherapy combined with neoadjuvant chemotherapy has recently become available for treating patients with triple-negative breast cancer, owing to increased rates of pathologic complete response.
Previous studies have explored the potential of DCE-MRI to help predict treatment response in breast cancer patients. These include using ultrafast protocols and also AI assistance, demonstrating the modality’s promise in breast imaging.
Rauch and colleagues evaluated whether DCE-MRI tumor volume changes that are measured early during immunochemotherapy could predict treatment response.
They calculated tumor volume reduction in 62 women with triple-negative breast cancer stages I-III. These reductions were based on DCE-MRI at baseline, after two cycles after immunochemotherapy, and after four cycles of immunochemotherapy. The researchers also correlated these reductions with surgical pathology outcomes.
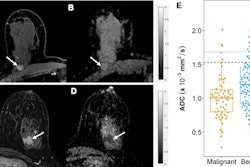
Of the total women, 38 (62%) achieved pathologic complete response after two cycles of immunochemotherapy. For the two-cycles evaluation, the team also reported that 86% of women with a tumor volume reduction of 90% or greater had pathologic complete response. Additionally, a tumor volume reduction of 35% or less predicted chemoresistance with a negative predictive value (NPV) of 100% and an area under the curve (AUC) of 0.71.
After four cycles, 82% of women with a tumor volume reduction of 95% or greater had pathologic complete response. Also, a tumor reduction volume of 75% or less predicted chemoresistance with an NPV of 100% and an AUC of 0.81.
For excellent responders, such prediction could mean de-escalation of treatment intensity while nonresponders can be referred to alternative treatment strategies.
Rauch said that these preliminary results will be validated in a larger cohort after the team completes its ongoing prospective clinical trial.