While mammography remains the unchallenged gold standard for breast imaging, there is increasing interest in developing alternatives to x-ray-based techniques. Such technologies could provide clinical information useful for cancer screening, without mammography’s radiation exposure and breast compression.
Breast imaging based on optical imaging is one such technology. Now, researchers at Clemson University in South Carolina have joined the race to develop breast imaging technology based on laser light. A Clemson research team has just completed phase I clinical trials for its first-generation optical tomographic device, and is working on a second-generation 3-D unit.
Developed by assistant physics professor Huabei Jiang and a team of postdoctoral fellows, Clemson’s system sends near-infrared laser light through breast tissue to photomultiplier tubes (PMTs) arranged in a 16-point array. The bundles of 3-mm PMTs measure light scatter at the breast's surface. Computer algorithms then reconstruct the data to produce images of the breast’s interior tissue.
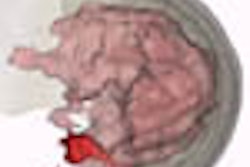
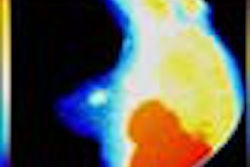
The scan takes place while the patient lies face-down on an exam table, onto which an adjustable-ring housing has been fitted. Software developed for the device analyzes the behavior of laser light as it travels through tissue, quantifying its absorption and scatter. The unit produces images in which the tumor is color-highlighted.
"The principle of laser imaging is that tumors scatter and absorb light differently than normal tissue," Jiang said. "Tumors create dense vascular beds around themselves, and when you shine infrared light on this kind of tissue, you can pick up on the increased vascularization."
Jiang's research in optical tomographic imaging began in 1993, when he was a graduate student in biomedical engineering at Dartmouth College in Hanover, NH. He began to investigate the interface between software and hardware for the technology, and in 1997 established the Clemson team after becoming a professor there.
Initially funded by the Greenville Hospital System/Clemson University Biomedical Cooperative, the project has also received about $600,000 from the National Institutes of Health’s National Cancer Institute and the Department of Defense’s breast cancer research program.
The Clemson team has built a first-generation prototype of the optical tomography device, with the optics mounted on a 1 x 1-m bench and the electronics on a moving cart. The next-generation device will have all its electronics and optics enclosed in casing around the exam bed, and will incorporate 64 detectors for 3-D imaging and four rings of fiberoptics instead of one.
This new incarnation will also reduce scanning time from 10 minutes to three minutes, Jiang said. The Clemson team is collaborating on the device with specialists at the Greenville Hospital System in Greenville, SC, and radiologists at the Medical University of South Carolina in Charleston. Phase I clinical trials were conducted at Greenville Hospital, and Jiang and his team hope to begin phase II trials in December, with at least 100 patients at multiple sites including Clemson and Greenville Hospital.
The team hopes that the optical tomography device will eventually be able to predict whether a tumor is benign or malignant. "It needs further study, but we think it might be possible to see a difference between benign and malignant tumors," Jiang said. "Preliminary images show a difference between the two in how the light scatters, but we have to continue to investigate why."
The Clemson researchers envision the device as an adjunct to traditional mammography, Jiang said, and believe that optical tomographic imaging could offer new screening possibilities for young, at-risk women without adding years of radiation exposure. The team would like to license the technology to a company that would take the device through the regulatory approval process and market it.
If the Clemson device does make it to market, chances are it won't be the only optical mammography system available. Commercial firms are also developing similar technology, notably Imaging Diagnostic Systems of Fort Lauderdale, FL. The company has begun taking preliminary orders for its CT laser mammography system in Europe, and is working through the FDA approval process in the U.S.
Whether optical tomographic laser imaging technology will ever replace mammography as the gold standard for breast cancer screening remains to be seen, according to Jiang.
"In a couple more years we will have completed statistically significant clinical trials that may confirm laser tomography’s use as a screening technology," he said. "We’re still at the clinical trial stage, but as a researcher, I’m optimistic. We believe that there are potential advantages to laser tomography that aren’t found in mammography."
By Kate Madden Yee
AuntMinnie.com contributing writer
April 27, 2001
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com