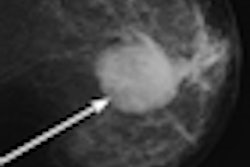
Accurate characterization of breast lesions should reduce the number of unnecessary biopsies. With angiogenic vasculature in such lesions indicative of malignancy, contrast-enhanced ultrasound imaging could provide a means for determining malignancy based on blood flow. Researchers from Thomas Jefferson University are investigating the use of subharmonic contrast-enhanced ultrasound imaging for this purpose.
"The ability to image vasculature in real-time, while completely suppressing all surrounding tissue signals, is exclusive to subharmonic ultrasound imaging," explained Flemming Forsberg, professor of radiology at Thomas Jefferson. "Isolation of signals generated within the blood supply allows us to image the vascular architecture of a lesion, which has been shown to be useful for classification."
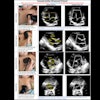
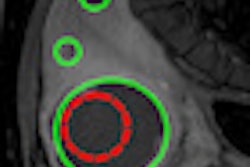
A pilot study revealed that cumulative maximum intensity (CMI) projections of subharmonic imaging (SHI) data provided higher diagnostic accuracy for breast lesions than mammography, but that CMI lacked a means of quantification. In a step toward quantitative lesion characterization, the research team has now published details of an algorithm that can extract vascular architecture in breast CMI-SHI (Physics in Medicine and Biology, February 21, 2011, Vol. 56:4, pp. 919-930).
Automated extraction
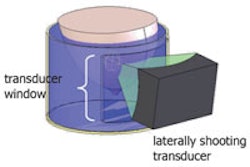
SHI involves transmitting ultrasound at double the resonance frequency (2f0) and receiving at the subharmonic (f0). Subharmonic emissions from injected microbubble contrast agents can distinguish vascular structures from tissues, which do not generate subharmonics. The CMI projection reveals the maximum intensity achieved at each pixel during flow of contrast, resulting in a map of lesion vasculature.
To enable automated extraction of critical parameters for quantification, these complex vascular images need to be thinned to create single line structures. The resulting "vascular skeletons" enable visualization of the basic architecture of the blood vessels.
The researchers tested three potential algorithms: a sequential thinning algorithm, a parallel thinning algorithm, and a distance transformation, using them to thin 40 binary test images. Results were compared based on qualitative parameters: connectivity and rotational invariance; and quantitative measures: data reduction efficiency and medial axis representation.
All three algorithms enabled skeletonization of all the test images, with connectivity retained. Images processed with the sequential thinning algorithm exhibited the lowest noise and the least rotational variance. Data reduction efficiency was also significantly better with sequential thinning than the other algorithms. The distance transformation method created the closest vascular skeleton to the ideal medial axis, with sequential thinning a close second.
The researchers concluded that, overall, sequential thinning was the superior algorithm, and used it to characterize a set of in vivo subharmonic breast images. CMI-SHI images were acquired from 16 breast lesions: four carcinomas and 12 benign lesions. Images were segmented with a threshold level of 10% (the best compromise between segmenting vasculature from background noise and maintaining vessel connectivity), and then the thinning algorithm applied to the segmented images.
Skeletonization was achieved for 13 of the 16 breast lesions. In the majority of these, well-defined vascular skeletons were revealed. The researchers computed the number of bifurcations (terminal ends within each skeleton) and average vessel chain length (skeletal pixels divided by bifurcations) for each image. Overall, the lesions showed an average of 7.6 bifurcations and an average vessel-chain length of 71.1 pixels/chain.
Malignant lesions had a higher number of bifurcations compared to benign lesions (9.8 versus 6.9) and a larger average vessel-chain length (88.9 versus 63.2 pixels/chain). While these differences were not statistically significant, they concur with the more aberrant vasculature expected in malignant tissue.
"It is well documented that malignant vasculature contains more tortuous, bifurcated blood vessels and overall a more chaotic vascular morphology," Forsberg explained. He noted that the skeletons can also reveal other parameters, such as vessel density, vascular curvature, and fractal dimensionality, which may prove useful for breast lesion characterization.
The researchers point out that this initial study was limited by a high degree of variability within the dataset and the small sample size. Applying the algorithm to a larger dataset should improve its ability to differentiate healthy from diseased tissue.
"Our group has recently received NIH funding for a 3D subharmonic breast ultrasound study in 450 women, to be conducted at Thomas Jefferson University and the University of California, San Diego," Forsberg told medicalphysicsweb. "This large 3D dataset will first determine the value of subharmonic imaging, and later be skeletonized to determine the method's ability to successfully characterize breast lesions."
By Tami Freeman
Medicalphysicsweb editor
February 18, 2011
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.