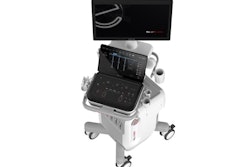
MyLab A70 ultrasound system.Image courtesy of Esaote
MyLab A70 ultrasound system.Image courtesy of Esaote
Esaote has received U.S. Food and Drug Administration (FDA) clearance to market its MyLab A50 and MyLab A70 ultrasound systems.
The systems include support for both routine diagnostics and advanced imaging, such as liver elastography and attenuation imaging, and cardiac ultrasound imaging using strain analysis, enabling detailed and multiparametric assessments, according to the firm.
Previously unveiled to the international medical community, the systems feature a conventional and touch control interface and compact design for flexibility across a wide range of clinical environments, according to Esaote.