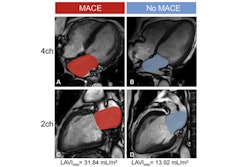
A machine-learning (ML) model could predict long-term major adverse cardiovascular events in patients with ST-segment elevation myocardial infarction (STEMI), suggest findings published February 17 in Radiology.
The model integrates cardiac MRI and clinical parameters and achieved “excellent” predictive performance compared with existing clinical risk scores and traditional models, highlighted a team led by WeiHui Xie, MD, PhD, from Shanghai Jiao Tong University in China and colleagues.
“Beyond its discriminative power, the model’s consistent performance across different clinical endpoints confirms its stability and broad clinical applicability,” the Xie team wrote.
While advancements in therapies and interventions have reduced STEMI-related mortality, patients still have a high risk of experiencing major adverse cardiovascular events (MACE). The researchers highlighted the need for improved risk stratification systems that can accurately predict MACE in patients with STEMI.
Prior studies suggest that cardiac MRI could help in this area by imaging cardiac morphologic features, cardiac function, and myocardial tissue activity in a single exam. Studies have also shown that several cardiac MRI-derived parameters have strong ties to adverse cardiac outcomes.
Xie and co-authors developed and externally tested their ML model by integrating clinical data and cardiac MRI parameters for long-term MACE prediction in patients with STEMI. Their study included data collected between 2015 and 2023 from 1,066 STEMI patients who underwent clinically indicated cardiac MRI within seven days of percutaneous coronary intervention.
Of the total patients, 682 were included in the training set, and 384 were in the external test set. During a median follow-up of 40 months, 142 patients in the training set and 81 in the external test set experienced MACE, respectively.
 Time-dependent receiver operating characteristic (ROC) curves for prediction of major adverse cardiovascular events (MACE) at (A) 12 months, (B) 24 months, (C) 36 months, and (D) 48 months. Across all evaluated time points, the machine learning (ML) model consistently achieved the highest area under the receiver operating characteristic curve (AUC) compared with the Global Registry of Acute Coronary Events (GRACE) score, Thrombolysis in Myocardial Infarction (TIMI) score, and Cox-based models, indicating robust and stable long-term predictive performance. RFE stands for recursive feature elimination.RSNA
Time-dependent receiver operating characteristic (ROC) curves for prediction of major adverse cardiovascular events (MACE) at (A) 12 months, (B) 24 months, (C) 36 months, and (D) 48 months. Across all evaluated time points, the machine learning (ML) model consistently achieved the highest area under the receiver operating characteristic curve (AUC) compared with the Global Registry of Acute Coronary Events (GRACE) score, Thrombolysis in Myocardial Infarction (TIMI) score, and Cox-based models, indicating robust and stable long-term predictive performance. RFE stands for recursive feature elimination.RSNA
The ML model achieved a higher integrated area under the receiver operating characteristic curve (AUC) for MACE prediction on the external test set. The team compared its model with a clinical model that used routine prognostic factors, the Global Registry of Acute Coronary Events score, and the Thrombolysis in Myocardial Infarction score.
Comparison between ML model, other risk models, and scoring systems | |
Model/scoring system | AUC |
ML model | 0.91 |
Clinical model | 0.86 |
Global Registry of Acute Coronary Events | 0.66 |
Thrombolysis in Myocardial Infarction | 0.62 |
| *The ML model achieved statistical significance compared to all other models and scoring systems. | |
The ML model also outperformed two Cox regression models. These included a traditional Cox model (AUC, 0.86) and a Cox model using the same features as the ML model (AUC, 0.89; p = 0.005 for both Cox models).
The ML model also showed high classification performance. This included 82.7% sensitivity, 84.5% specificity, and 84.1% overall accuracy. And the model effectively stratified patients into distinct risk groups (log-rank, p < 0.001).
The study authors highlighted the model’s “strong potential” for personalized risk assessment. They also called for future studies to focus on populations in other countries and to incorporate more accessible imaging modalities like echocardiography.
“Additionally, to ensure broader clinical adoption, future work should focus on developing simplified models with fewer features while maintaining accuracy and integration into routine workflows,” they added. “Finally, the clinical value of ML needs further exploration before it can be effectively integrated into clinical practice.”
Despite the model's predictive accuracy, prospective studies are needed to examine patient outcomes and medical costs, according to an accompanying editorial written by Jérôme Garot, MD, PhD, and Suzanne Duhamel, MD, both from Cardiovascular Institute Paris Sud in Massy, France.
"As a community, we must determine soon whether the use of such 'fuzzy but attractive' technology is worth it in terms of patient outcomes and medical and economic efficiency," Garot and Duhamel wrote.
Read the full study here.