PET/MRI scans show that a promising new treatment for neurological and psychiatric conditions works by triggering an increase in dopamine, researchers in France have reported.
The finding supports the use of what’s called transcranial direct current stimulation for patients with dopamine dysregulations, such as schizophrenia, noted lead author Clara Fonteneau, PhD, of Claude Bernard University Lyon 1, and colleagues.
“This study provides new insights into the neurophysiological mechanisms of frontotemporal tDCS, paving the way for the optimization of therapeutic strategies for patients,” the group wrote. The study was published May 5 in Brain Stimulation.
Transcranial direct current stimulation (tDCS) is a promising noninvasive intervention for treatment-resistant schizophrenia, particularly when applied using a frontotemporal montage, the researchers explained. The technique consists of delivering a weak electrical current through at least two electrodes placed over the scalp of a participant to modulate brain activity of targeted regions.
Although significant clinical benefits have been reported, variability in individual responses highlights a need for a more comprehensive understanding of its underlying mechanisms, the group noted. Hence, in this study, the researchers used simultaneous PET/MRI to investigate the effects of frontotemporal tDCS on dopamine transmission, cerebral perfusion, and white matter microstructural integrity in healthy individuals.
During a single experimental visit, 30 participants underwent a 30-minute session of frontotemporal tDCS while simultaneously undergoing a PET/MRI scan (Biograph mMR, Siemens Healthineers). The group were randomly allocated to receive a single session of either active (n=15) or sham (n=15) frontotemporal tDCS.
PET imaging assessed dopamine transmission with the radiotracer carbon-11 (C-11) raclopride, while MRI included anatomical T1-weighted scans, arterial spin labelling (ASL) for cerebral blood flow, and diffusion weighted imaging (DWI) for microstructural white matter integrity.
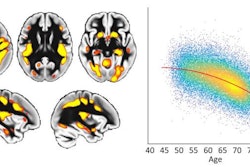
 Changes in C-11 raclopride non-displaceable binding potential (BPND) in the left executive striatum during and after frontotemporal tDCS in the active and sham groups. The curves show the variation in BPND across five time points: Baseline (25-40 min), Stim1 (40-55 min), Stim2 (55-70 min), Post1 (70-85 min) and Post2 (85-100 min) for the active group (red line) and the sham group (blue line). A significant decrease in BPND, indicating dopamine release, is observed at Post2 in the active compared with the sham group. Error bars represent the standard error of the mean. # indicates significant Group by Time interaction; * indicates significant post hoc differences. Executive, limbic, and sensorimotor striatal subregions are illustrated for reference as defined by the Oxford-GSK-Imanova connectivity striatal atlas.Brain Stimulation
Changes in C-11 raclopride non-displaceable binding potential (BPND) in the left executive striatum during and after frontotemporal tDCS in the active and sham groups. The curves show the variation in BPND across five time points: Baseline (25-40 min), Stim1 (40-55 min), Stim2 (55-70 min), Post1 (70-85 min) and Post2 (85-100 min) for the active group (red line) and the sham group (blue line). A significant decrease in BPND, indicating dopamine release, is observed at Post2 in the active compared with the sham group. Error bars represent the standard error of the mean. # indicates significant Group by Time interaction; * indicates significant post hoc differences. Executive, limbic, and sensorimotor striatal subregions are illustrated for reference as defined by the Oxford-GSK-Imanova connectivity striatal atlas.Brain Stimulation
According to the results, C-11 raclopride PET revealed a significant reduction in non-displaceable binding potential in the left executive striatal subregion 15 minutes after tDCS in the active group, compared to both baseline and the sham group.
“This finding suggests that frontotemporal tDCS may induce an increase in dopamine release,” the group wrote.
In addition, ASL analysis showed that active tDCS may reduce cerebral blood flow in the precuneus compared to sham stimulation, while no significant effects of tDCS were observed on white matter microstructural integrity, the researchers reported.
Ultimately, the study provides the first evidence that frontotemporal tDCS induces dopamine release in polysynaptically connected subcortical regions, particularly the left executive striatum, and modulates cerebral blood flow in the precuneus, the researchers wrote.
“Further research is necessary to determine whether the observed effects can be translated to individuals with dopamine dysregulation or altered brain connectivity in the frontotemporal network, such as patients with schizophrenia,” the group concluded.
The full study is available here.