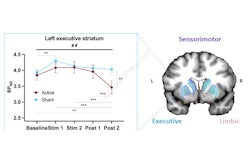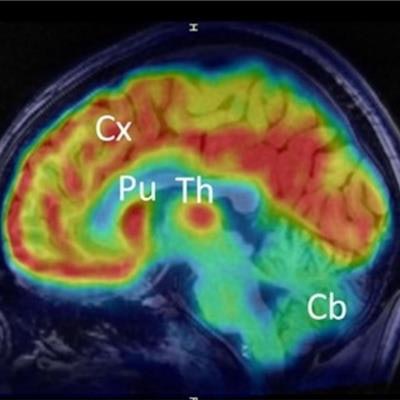
Researchers are highlighting a new PET radiotracer that they say can improve clinical neuroimaging in a June 25 presentation at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Chicago.
In his presentation, Steven Liang, PhD, from Emory University in Atlanta discussed the team's findings, which showed that their radiotracer, known as F-18 Cholestify, successfully images enzymes tied to metabolic cholesterol degradation in the brain.
"We hope clinicians can utilize it for study of cholesterol changes during neurological disorders and neurodegenerative diseases.," Liang told AuntMinnie.com.
The enzyme CYP46A -- also known as cholesterol hydroxylase -- is responsible for eliminating excessive brain cholesterol. Neurodegenerative diseases and neurological disorders such as Alzheimer's disease impact the enzyme's functioning and in turn, brain cholesterol metabolism.
While quantitative mapping of the enzyme could aid in diagnosis and treatment strategy, Liang pointed out that no validated methods exist to visualize metabolic cholesterol clearance in the brain.
Liang and colleagues developed a CYP46A1-targeted PET radioligand with high CYP4A1 binding affinity. For F-18 Cholestify, they radiolabeled the molecule CHL2205 with F-18.
The researchers wanted to put their radiotracer to the test by determining the molecular strength of CHL2205 in rodent models, nonhuman primate models, and healthy human volunteers via radioligand saturation and competitive binding assays. The team performed autoradiographies on rodent brain tissue and carried out PET imaging studies on rodents, nonhuman primates, and human volunteers.
For preclinical evaluation, the researchers found that F-18 Cholestify demonstrated "remarkable" selectivity for CYP46A-rich brain regions in the rodent models. These regions include the striatum (0.2), cortex (0.3), thalamus (0.27), and striatum (0.36). However, they also found that cerebellar uptake was low in both in vitro and in vivo models.
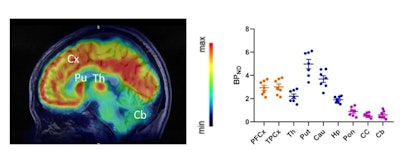 Clinical translation of F-18-Cholestify in healthy volunteers showed representative PET and non-displaceable binding potential in humans. The graph shows correlations of regional PET signal (SUV) with nondisplaceable binding potential values and Western blot analysis of CYP46A1 from post-mortem brain specimens. Images courtesy of the SNMMI.
Clinical translation of F-18-Cholestify in healthy volunteers showed representative PET and non-displaceable binding potential in humans. The graph shows correlations of regional PET signal (SUV) with nondisplaceable binding potential values and Western blot analysis of CYP46A1 from post-mortem brain specimens. Images courtesy of the SNMMI.Additionally, they confirmed regional selectivity for CYP46A1-rich brain areas in nonhuman primate PET studies and in a first-in-human study. They also found through kinetic modeling that the PET signal had "excellent" correlation with non-displaceable binding potentials and with Western-blot results in post-mortem specimens in nonhuman primates.
Specifically, the radioligand binding assays with nonhuman primate brain homogenates showed "slightly" higher dissociation constants of 0.82 nanometers (nM) for the putamen region of the brain and 1.21 nM for cerebellar brain homogenates.
"This is a textbook example of neuroPET ligand development and we have validated the ligand in rats, nonhuman primates and humans to confirm its high specificity," Liang said. "An interesting observation, yet not surprising, is that we found sex differences in the cholesterol metabolism between men and women. Women showed statistically significant higher brain uptake."
The team suggested that their findings point to F-18-Cholestify PET imaging being eventually integrated into clinical studies investigating neurodegenerative diseases and neurological disorders.
Liang said the team will next transition this imaging tool into studies including schizophrenia, ALS, Alzheimer's disease, and Huntington's disease. He told AuntMinnie.com that the team hopes that pharmaceutical companies can develop novel modulators for treating cholesterol-related brain disorders.