The U.S. National Institutes of Health (NIH) has highlighted a study featuring PET imaging of patients treated with denosumab, a new drug for a rare bone disease called fibrous dysplasia.
In a clinical trial in eight women, PET scans revealed that denosumab significantly reduced abnormal bone turnover in adults with fibrous dysplasia.
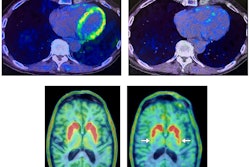
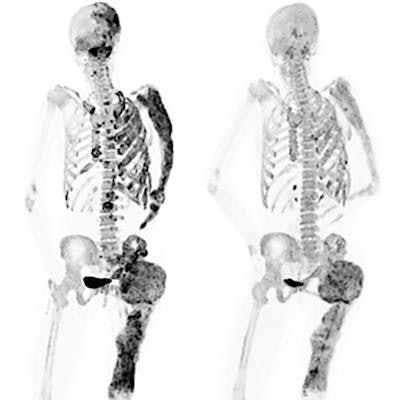
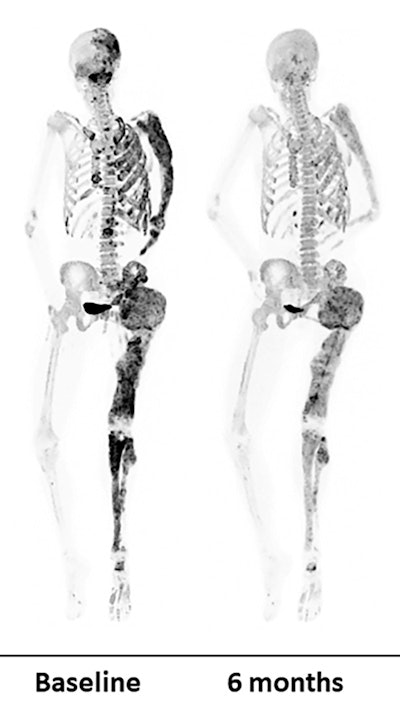 PET bone scans of a patient before (left) and after (right) a six-month denosumab treatment show reduced turnover within fibrous dysplasia lesions (dark-colored patches). Image courtesy of Dr. Alison Boyce, NIDCR.
PET bone scans of a patient before (left) and after (right) a six-month denosumab treatment show reduced turnover within fibrous dysplasia lesions (dark-colored patches). Image courtesy of Dr. Alison Boyce, NIDCR.Bone turnover is a process in which old bone is continuously replaced with new bone and this process is unusually accelerated in fibrous dysplasia. In some cases, abnormalities can press up against organs and nerves, impairing functions like vision and breathing.
Surgery is still the standard treatment for fractures and deformities caused by fibrous dysplasia, noted lead author Dr. Alison Boyce, a clinical investigator at the National Institute of Dental and Craniofacial Research (NIDCR).
"Denosumab is the first medication that appears to affect how fibrous dysplasia lesions behave and improves patients' disease outcomes," she said in an April 4 news release.
In the phase II clinical trial, researchers performed PET/CT scans using F-18 sodium fluoride (NaF) radiotracer in patients at baseline and again six months after receiving high doses of denosumab. While the imaging suggests denosumab shows promise, abnormal bone turnover returned for all but one of the patients after they stopped taking the medication, Boyce noted. In four patients, bone turnover exceeded pretreatment levels.
To determine whether earlier intervention with denosumab may prevent lesions from forming altogether, Boyce and her team are now testing the medication in a new clinical trial for children with fibrous dysplasia, according to the release.
The small study offers significant implications for this patient group, added Dr. Rena D'Souza, PhD, director of NIDCR.
"This trial is the culmination of 25 years of clinical research at NIDCR to understand the underlying mechanisms of fibrous dysplasia and to identify promising treatments," he stated.
Results of the trial were published in a February 23 correspondence in the New England Journal of Medicine.