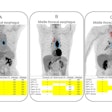
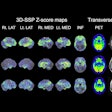
PET technology developer Prescient Imaging said it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its BBX-PET portable PET scanner.
Designed to be wheeled to the point of care, BBX-PET can provide PET imaging of the brain, breast, and extremities, according to the vendor. It can be used for research, diagnosis, therapeutic planning, and therapeutic outcome assessment, the firm said.
The company believes BBX-PET will be useful for hospitals and imaging centers, as well as neurology, geriatric, psychiatry, and radiation oncology centers. Prescient Imaging said that its scanners incorporate the claims of three issues and two pending U.S. patents for which it owns or has exclusive licenses.