The U.S. Food and Drug Administration (FDA) issued a letter July 26 reminding healthcare providers about the intended use of radiological computer-aided triage and notification (CADt) devices.
"Health care providers may not be fully aware that [large vessel occlusion] CADt devices are intended for prioritization and triage only," the FDA's letter stated.
The reminder follows the publication of an FDA-funded study that suggests providers may not be aware of the intended use of these devices, the FDA said.
According to the FDA, large vessel occlusion CADt devices are software devices intended to aid in prioritization and triage of time-sensitive suspected findings of large vessel occlusion based on the analysis of radiological exams of the brain. They do not provide diagnostic information or remove any cases from the imaging physician's reading queue, the letter stated.
"If [large vessel occlusion] CADt devices are not used as intended, there is the potential for misdiagnosis resulting in patient injury or death," the FDA said.













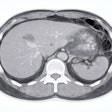
![Images show the pectoralis muscles of a healthy male individual who never smoked (age, 66 years; height, 178 cm; body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], 28.4; number of cigarette pack-years, 0; forced expiratory volume in 1 second [FEV1], 97.6% predicted; FEV1: forced vital capacity [FVC] ratio, 0.71; pectoralis muscle area [PMA], 59.4 cm2; pectoralis muscle volume [PMV], 764 cm3) and a male individual with a smoking history and chronic obstructive pulmonary disorder (COPD) (age, 66 years; height, 178 cm; BMI, 27.5; number of cigarette pack-years, 43.2, FEV1, 48% predicted; FEV1:FVC, 0.56; PMA, 35 cm2; PMV, 480.8 cm3) from the Canadian Cohort Obstructive Lung Disease (i.e., CanCOLD) study. The CT image is shown in the axial plane. The PMV is automatically extracted using the developed deep learning model and overlayed onto the lungs for visual clarity.](https://img.auntminnie.com/mindful/smg/workspaces/default/uploads/2026/03/genkin.25LqljVF0y.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&h=112&q=70&w=112)