Staging and its Effect on Treatment and Prognosis
Although the staging system applies to all forms of bronchogenic carcinoma, from a clinical and therapeutic standpoint lung cancer is divided into two histologic categories: small cell lung cancer and non-small cell lung cancer (NSCLC). Small cell lung cancer accounts for between 15-25% of all malignant lung tumors and it is considered to be a systemic disease best treated with chemotherapy [6]. Some patients with limited disease at presentation will go to surgery, but post-operative chemotherapy is used in these patients as micrometastases are suspected to be present in most patients [6]. Because of this general consensus regarding small cell lung cancer, the discussion below focuses on the staging, treatment, and prognosis of patients with non-small cell lung cancer.
In general, for patients with NSCLC earlier stage at the time of diagnosis, female sex, and younger age are associated with a better prognosis [195]. For specific cell types, patients with pure or mixed bronchoalveolar cell cancers have the best survival across all calculations [195]. Patients with squamous cell carcinomas also seem to have a survival advantage compared to patients with adenocarcinoma and large cell carcinoma [195].
Complete surgical excision, combined with systematic and meticulous mediastinal node dissection is the standard procedure for lung cancer operations, and is defined as a radical resection or a curative operation when no residual tumor remains after operation. Pulmonary resection without mediastinal lymph node dissection or pulmonary resection with residual tumor remaining after the operation is a palliative operation [6,7]. Overall surgical mortality following resection of lung cancer is 3.7%, however, mortality is higher following pneumonectomy and in patients over the age of 70 years [6].
There is much variability in the literature regarding patient survival following surgical resection for bronchogenic carcinoma. The technical considerations and approaches of surgeons vary and a mass considered unresectable by one surgeon, may be considered technically resectable by another [8]. Factors such as the heterogeneity of the patients studied, variations in the extent of resection, difficulty in comparing survival data on patients from different institutions who are treated by different surgeons, various regimens of post-operative care (radiation or chemotherapy), and the lack of consensus about inclusion criteria limit the conclusions that can be drawn from many studies [8,9].
In patients that are not surgical candidates, radiofrequency ablation can be used for treatment of lung neoplasms [156]. The treatment is generally well tolerated and requires only a short hospital stay [156]. Complications include pneumothorax (17%), pleural effusion (30%), fever (46%), and some mild hemoptysis [156]. The procedure is best performed under general anesthesia with a double lumen endotracheal tube- this makes it possible to block ventilation to the lung being treated which allows accurate needle placement [156].
Stage
In non-small cell lung cancer the main therapeutic approach is surgery for stage I and II disease-- however, this subgroup constitutes only a minority (20-25%) of patients. Patients with Stage IIIA disease may be candidates for surgical resection, and some studies have even evaluated the benefit of surgery in certain patients with Stage IIIB disease [2], although this group is generally considered non-resectable. Patients with Stage IV disease are not candidates for surgical resection, although patients with an isolated brain metastasis and otherwise resectable primary lesion, may be an exception at certain institutions.
Unfortunately, the majority of patients with NSCLC initially present with locally advanced or metastatic disease [2]. Estimates of the percentage of patients whose disease is not resectable at presentation vary from 65% to 80% [4]. This helps to explain why despite advances in treatment and surgical resection the overall 5 year survival has increased only from 8% to 13% in the last 25 years [99].
Stage I
IA: T1a-T1bN0M0
IB: T2aN0M0
Stage I lesions are considered
surgically resectable. Lobectomy has provided a 5-year survival
of 61% for patients with clinical stage IA disease, and 38% for
those with clinical stage IB. Survival based upon
surgical-pathologic staging reveals a 5-year survival of 67-80%
for stage IA, and 57-65% for stage IB lesions [3,118,119,173].
Even among Stage IA patients, however, lesion size can affect overall patient survival [157]. Five year disease free survival can be as high as 77-81% for tumors less than 2 cm, but decreases to 63-71% for lesions over 2 cm, but less than or equal to 3 cm [157,192]. Hence, the new staging system reflects these differences in survival [192,197]. Cavitation of a stage I lesion is associated with an adverse prognosis [163]. Preoperative chemotherapy may offer a survival benefit in patients with early stage lung cancer, but further studies will be required [148].
Lobectomy has been considered the standard of care for patients with stage I primary lung cancer. Older studies suggested a 15% to 20% survival benefit in patients who undergo resection of the tumor with lobectomy, rather than a segmental resection [6], although the survival benefit may primarily affect patients with primary lesions larger than 3.0 cm [10]. A more recent study suggested a non-significant trend toward better survival following lobectomy- with greater significance for lesions less than 3.0 cm [176]. Some studies also indicate that lesser pulmonary resections for T1N0 lesions are associated with a higher risk of local recurrence [96]. However, others report excellent 5-year survival following limited resection for stage IA tumors less than 2 cm [194] with an overall 5-year survival rate of 89% [201]. The use of adjunctive intraoperative brachytherapy may also aid in decreasing the risk for local recurrence following sublobar resection [194]
None-the-less, wedge resection may be considered as a viable alternative in patients that are not surgical candidates for more extensive resections [107]. In fact, a 2005 meta-analysis suggested comparable survival between the two procedures, although interstudy heterogeneity limits a definitive conclusion [177]. Limited surgical resection is also an option for patients with minimally invasive adenocarcioma and part-solid nodules with greater than 50% ground glass component [193]. In these cases, radical segmentectomies with hilar and mediastinal lymph node dissection or sampling only still results in high curability rates [193].
Another factor to consider is that among patients who survive one lung cancer, there is a 2.5% annual risk of developing a second primary lung cancer [157]. With earlier cancer detection, the more lung that is preserved during the first resection the greater opportunity for surgical resection of metachronous primary tumors [157]. For certain frail or high risk patients, lobectomy can be performed through the use of minimally invasive video-assisted thoracic surgery (VATS) [117].
Stage II
IIA: T1a-T1bN1M0, T2bN0M0, and
T2aN1M0
IIB: T2bN1M0 and T3N0M0
Stage II lesions are surgically
resectable, but fewer than 10% of the patients with non-small
cell lung cancer are found to have metastases confined to N1
nodes at presentation [6]. Based upon clinical staging, 5
year survival is 34% for stage IIA, and 24% for stage IIB
(T2N1M0). Clinical survival rates for patients with T3N0M0
tumors were 22%, which supported the rationale for changing the
designation of these lesions to stage IIB from stage IIIA tumors
[3]. Patients in whom stage II disease was identified at surgery
had an overall improved survival of 55-57% for stage IIA, 39-47%
for stage IIB (T2N1M0), and 34-38% for stage IIB (T3N0M0)
[3,118,119]. For patients with stage II disease, improved
survival has been reported in patients with squamous cell cancer
and N1 disease when compared to patients with adenocarcinoma
[6]. Because of decreased survival in previously classified T2
tumors larger than 7cm (35% at 5 years), these lesions are now
considered T3 [192]. A satellite tumor nodule in the same
primary tumor lobe is now classified as T3 [197]. Previously,
a satellite tumor nodule in the same primary lobe was classified
as T4, but the 5-year survival in these patients is 28%- which is
similar to the 31% survival for other T3 lesions [195,197].
Visceral pleural invasion leads to upstaging of even small lung
cancers from T1 to T2 [216]. Visceral
pleural involvement has been found to have an adverse effect on
overall 5-year survival in node-negative non-small cell lung
cancers measuring 3 cm or smaller [216]. Visceral
pleural invasion is suggested on CT by a direct pleural contact
length greater than one-fourth of the circumference of the solid
portion of the tumor, pleural retraction, or a pleural tag (thin
band/bands extending from a tumor to the pleural surface) [216].
In one study of patients with adenocarcinoma, CT features for
visceral pleural invasion had an accuracy of 63-72%, and a
positive predictive value of 44-56% [216].
Adjuvant chemotherapy based on cisplatin in combination with vinorelbine or gemcitabine is recommended for nodal-positive stage IIA-IIB disease [213]. Observation is recommended for node negative patients, unless one associated high risk factor is present including poorly differentiated tumor, vascular invasion, wedge resection, tumor > 4 cm, visceral pleural involvement, and unknown node status [213]. Volume based PET metrics (metabolic tumor volume and total lesion glycolysis) demonstrating a large metabolically active tumor may also be considered a risk factor for further treatment following resection [213]. Preoperative chemotherapy may offer a survival benefit in patients with early stage lung cancer, but further studies will be required [148]. Recurrence is more likely to be local in patients with squamous cell carcinoma, while systemic recurrence is more likely in patients with adenocarcinoma [6]. Post-operative radiation therapy may decrease the risk of local recurrence, but does not impart a survival benefit [6]. Recent data suggest a modest improvement in survival with the addition of adjuvant chemotherapy [179].
Stage IIB T3 lesions: T3N0M0
The surgical approach to patients with T3 tumors is controversial. Most studies suggest patients with parietal pleura or chest wall involvement benefit from a full-thickness chest wall surgical resection. However, the presence of metastases to regional lymph nodes, incomplete resection, and invasion beyond the parietal pleura are associated with a worse prognosis following resection [6,116]. The benefit of surgical resection is less clear for patients with localized mediastinal invasion or other T3 lesions [94,119]. Patients with T3 lesions involving the diaphragm have been shown to have a poor prognosis and some author suggest these should actually be considered T4 lesions [118,119,127].
For patients with chest wall invasion, 5-year post complete resection survival rates are between 30% to 49% when regional lymph nodes are negative [6,116,118]. The 5-year survival rate reported by the American Joint Committee on Cancer was 38% for pathologically staged T3N0M0 lesions (now classified as stage IIB) [3].
Superior sulcus tumors (or Pancoast tumors) are an uncommon subset of T3 tumors which arise in the lung apex. They account for less than 5% of all bronchogenic carcinomas [11,183]. In current reports, adenocarcinoma accounts for about two thirds of cases, and squamous cell cancers make up most of the remainder [12]. The Pancoast syndrome results from local extension into the chest wall and rib which produces shoulder pain (the most common presenting symptom [13]); involvement of the brachial plexus causing arm pain, weakness, and paresthesias (particularly in the ulnar nerve distribution); and spread to the sympathetic stellate ganglion leading to Horner's syndrome. Symptoms of Horner's syndrome are found in about 20% of patients [12] and include ptosis of the upper eyelid, miosis (pupillary constriction), and anhydrosis (absence of sweating); as well as constriction of the pupil, enophthalmos (sinking in of the eyeball), narrowing of the palpebral fissure (due to paralysis of the M?ller muscle [183]), and flushing of the affected side of the face. Patients with Horner's syndrome have a worse prognosis, but this is not a contraindication to surgery [12].
The following criteria usually signal a poor prognosis and generally indicate an unresectable lesion:
Absolute contraindication:
1- Bracheal plexus invasion at a level above the T1 nerve [183]
2- Greater than 50% invasion of a vertebral body [12,164,183]
3- Invasion of the trachea, or esophagus, or invasion of the soft tissues at the base of the neck [13,183]
4- Distant metastases [11,183]
5- Mediastinal lymph node (N2) or contralateral supraclavicular lymph node metastases [12,183]. Mediastinal lymph nodes mets are found in up to 20% of patients with superior sulcus tumors and mediasitnal staging is advised in all patients [183].
6- Phrenic or recurrent laryngeal nerve paralysis
Relative contraindications to surgical resection:
1- Tumor invasion of the subclavian vessels [12]-- most commonly the subclavian artery because of it's location
2- Vertebral body invasion of less than 50% [183]
3- Extension of the tumor into the spinal canal - intraforamenal extension [164,183]
4- Invasion of the common carotid or vertebral artery [183]
5- N1 (ipsilateral hilar) or N3 (ipsilateral supraclavicular) nodal metastases [183]
Limited involvement of the lower trunk or roots of the brachial plexus (C8 and T1 nerves) is generally regarded as T3 disease {183]. Resection of the lower cords of the brachial plexus (if involved) may be necessary, but extensive involvement (C5 through C7 nerves) usually renders the patient inoperable (T4 disease) [183]. Patients with vertebral body invasion have demonstrated improved survival following vertebral resection with reconstruction if negative margins can be achieved [164]. There is some evidence that patients with ipsilateral supraclavicular node (N3) involvement have a slightly better prognosis than patients with ipsilateral mediastinal node involvement (N2), probably because ipsilateral supraclavicular node involvement represents "local" tumor extension [12]. Despite this fact, overall 5 year survival is poor (approximately 14%) and pre-operative mediastinoscopy should be used for planning appropriate treatment. It may be best to treat patients with N2 to N3 disease (mediastinal or contralateral lymph node metastases) with irradiation or chemoradiation therapy [12,183]. MR imaging is the optimal modality for evaluating superior sulcus tumors given its ability to accurately depict chest wall invasion, extension into the neurovertebral foramina and spinal canal, and involvement of the brachial plexus [183].
For resectable lesions, multimodality therapy is the treatment of choice [183]. Preoperative radiation therapy is generally given to reduce primary tumor size. Typically 30-45 Gy is administered 3 to 6 weeks prior to surgery. Surgery involves en bloc resection of the chest wall, which may be accompanied by resection of the posterior portions of the upper ribs, portions of the upper thoracic vertebral bodies, the intercostal nerves, the lower trunks of the brachial plexus, the stellate ganglion, a portion of the dorsal sympathetic chain, the subclavian artery, and a lobectomy of the involved lung [13]. In addition, a careful mediastinal lymph node dissection is carried out. In some centers any residual tumor is implanted with iodine-125 seeds [6]. Surgery is associated with high morbidity (7-38%) and mortality (5-10%) [13]. The addition of chemothearpy in the management of patients with superior sulcus tumors has also been associated with prolonged survival [183]. A five year survival of 26-54% has been reported for patients receiving multimodality treatment [183]. Improved survival is associated with a lower stage at presentation (47% stage IIB vs 14% IIIA), weight loss of less than 5% of body weight before treatment, and surgical resection (in node negative patients) [183].
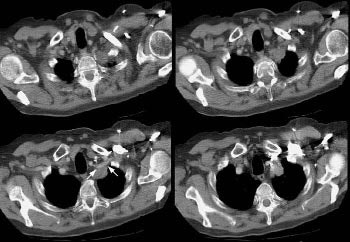
Superior sulcus (Pancoast) tumors:
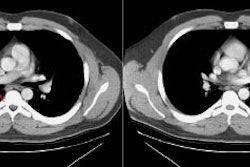
Example 1: This is an example of a superior sulcus tumor. The relationship of the lesion to the left subclavian artery is nicely demonstrated (white arrows).
.
NOTE: To load a higher resolution view, simply click directly on the image
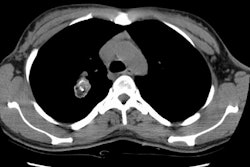
Example 2: This patient with a squamous cell superior sulcus tumor demonstrates obvious rib destruction and mass invading the chest wall (black arrows). There is loss of the tissue plane between the mass and the mediastinum and along the anterior aspect of the vertebral body which suggests mediastinal invasion as well (T4 lesion).
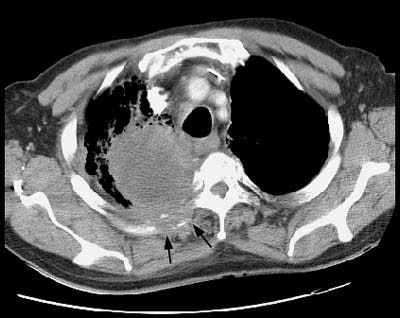
Stage III
IIIA: T1N2M0, T2N2M0, T3N1M0, T3N2M0, and T4N0-N1M0
IIIB: (Any T)N3M0, T4N2-N3M0
The five-year survival for patients with clinical stage IIIA disease is 9% to 13%, while pathologic stage IIIA patients had a survival between 23% to 25% [3]. Five-year survival for patients with clinical IIIB lesions was 3% to 7% [3]. Although these lesions are generally felt to be non-surgical, in certain instances resection can be considered- particularly for higher T-stage lesions with N0 or N1 node disease [112].
T4 N0 Disease:
Based upon survival data, some authors also suggest that patients with small synchronous tumor nodules in the same lobe as the primary tumor and no evidence of metastatic disease (T4N0M0) should not be considered Stage IIIB lesions [173] and that these patients should be considered for resection if there are no other contraindications to surgery due to their significantly better survival [113,173]. In one study, T4N0M0 satellite nodule patients had a 5 year survival of 57%- which was significantly better than patients with T4N0M0 lesions with vital structure invasion (30% 5-year survival) [173]. In another study of T4 N0 satellite nodule patients (excluding patients with BAC) the estimated overall 5 year survival was 40% [189]. That same study also evaluated other T4 satellite nodule patients- when stratified for node status, the median survival of node negative patients was about 35 months, compared to 20 months for N1 disease and 23 months for N2 disease [189]. T4 N0 satellite nodule patients with large lesions (over 4 cm), positive lymph node involvement, and non-adenocarcinoma histology appear to be at higher risk for worse outcomes [189].
N2 Disease in Stage III Non-small Cell Lung Cancer (NSCLC):
Metastases to mediastinal lymph nodes are found in nearly half of all patients with NSCLC [6], and patients with adenocarcinoma may be at an increased risk for the presence of unsuspected N2 node disease [15]. Unfortunately there is a large variation in the literature on survival and prognostic factors in patients with N2 lung cancer treated by primary surgical resection.
For patients with metastases to mediastinal lymph nodes confirmed by preoperative mediastinoscopy, it is generally agreed that these patients do not benefit from primary surgical intervention [186]. The 5-year survival has been reported to be 7% to 18% [7,15]. Patients with a pre-operative abnormal mediastinoscopy are preferably entered into a multimodality treatment plan, including induction chemotherapy, radiation therapy, or both, followed by potential surgery if there is no residual mediastinal disease [15,16,97,125,126,159,186]. A 4-year survival rate as high as 46% has been reported in patients following pre-operative chemoradiation and a complete surgical resection [2].
Primary surgical resection is still worthwhile if unsuspected N2 nodes are found at thoracotomy after a normal pre-operative mediastinoscopy [17]. In these cases, survival has been reported to be 14% to 32% [6,7,15,18]. Improved 5-year survival in these patients following resection has been described in association with a lower T-stage, squamous cell carcinoma cell type, fewer metastatic lymph node levels present (involvement of only one mediastinal nodal station), the absence of extracapsular extension, and a complete resection/curative mediastinal lymph node dissection [6,7,15,18,186].
Post-operative radiation may offer an added survival benefit in certain of these patients deemed to be at increased risk for local recurrence [16]. Post-operative chemotherapy has not been shown to result in increased patient survival [15], although pre-operative chemotherapy (with post-operative radiation therapy) [9] or combined pre-operative chemoradiation therapy [2] may offer a survival benefit in select patients with N2 stage III disease. Although it is generally agreed that adjuvant therapy can decrease local recurrence and improve disease free survival, it is not universally accepted that these treatments have a statistically significant effect on overall survival [97].
Non-resectable IIIA and IIIB disease:
In patients with stage IIIA or IIIB
disease that is unresectable, either surgically or because the
patient is inoperable, combination chemotherapy and radiation
therapy offer the best survival benefits [97,123].
Patients with Stage IIIB lesions and malignant pleural effusions
(T4 lesion) have significant worse survival than IIIB patients
without effusions (survival is similar to patients with stage IV
disease) [113]. As a result, some authors feel that it would be
more appropriate to classify the presence of a malignant pleural
effusion as stage IV disease [113].
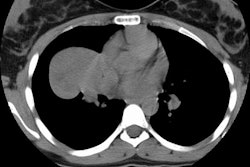
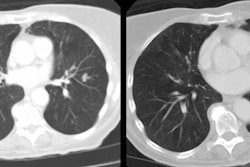
Example: The images below illustrate a case of non-small cell lung cancer which involved the carina (see drawing at right). Although this is typically considered a non-resectable T4 lesion, the tumor was treated surgically with a right carinal pneumonectomy due to the patient's young age. (Case courtsey of Dr. Douglas E. Wood, M.D.).
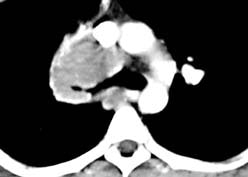
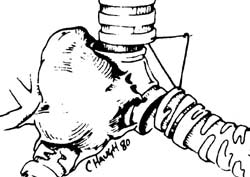
Stage IV
(Any T)(Any N) M1
Five-year survival for patients with Stage IV disease is about 1% [3]. Patients with stage IV lung cancer are not considered surgical candidates. Despite the generally accepted lack of surgical options for patients with Stage IV disease, an exception to this policy may be the patient with an otherwise resectable primary tumor and a solitary brain metastasis presenting synchronously or metachronously. Excision of the primary lesion and the brain metastasis with post-operative radiation therapy to the brain can occasionally improve survival [4,6].
Synchronous Lung Tumors:
A physically distinct and separate
lung tumor with a different cell type is considered a
synchronous primary lesion and should be staged independently.
Separate lesions with the same histology are more difficult to
characterize. They may be considered synchronous if there is no
evidence of carcinoma in the lymphatics common to both and no
other evidence for metastatic disease exists [134]. Synchronous
primary lesions account for less than 1% of lung cancer
presentations [113]. Optimum therapy is surgical resection of
each lesion, but patients still have an overall worse prognosis
than that expected from a single tumor of a similar stage
[113,134].