Semin Nucl Med 1993 Apr;23(2):89-98
Imaging of colorectal carcinoma with technetium-99m radiolabeled Fab'
fragments.
Podoloff DA, Patt YZ, Curley SA, Kim EE, Bhadkamkar VA, Smith RE.
In this phase III study, patients who had previously undergone surgery for colorectal
cancer were studied using a technetium-99m (99mTc)-labeled anti CEA antibody (IMMU-4
[Immunomedics, Morris Plains, NJ] 1mg of protein) to evaluate recurrence. Total-body,
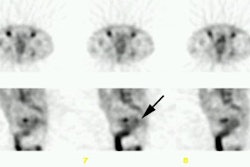
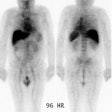
planar, and single photon emission computed tomography (SPECT) images were performed
within 6 hours of injection. Objectives were to evaluate the efficacy of the 99mTc-labeled
anti-CEA antibody, to assess sensitivity and specificity of the agent in known lesions,
and to detect occult disease. The impact of antibody study on subsequent surgery was also
evaluated. The Fab' fragment has a molecular weight of 54,000 and is supplied as a
lyophilized kit that can be instantaneously labeled with 20 to 30 mCi of
[99mTc]pertechnetate. In 9 patients with known disease, planar spot imaging identified
lesions in 7 (78% sensitivity), SPECT imaging detected lesions in 8 (88% sensitivity), and
1 patient did not have SPECT. In the group of 10 patients with occult (or equivocal)
disease, planar imaging sensitivity was 50%, and SPECT sensitivity was 100%. Analysis by
site showed 14 of 24 lesions detected by planar imaging (58% sensitivity), and SPECT
detected 24 of 24 lesions (100% sensitivity). Tumors as small as .5 cm were visualized in
the 19 patients studied. The surgeon judged the antibody study to be impact neutral in 73%
of the cases and helpful in 27% of the cases when antibody study altered the presurgical
plan.
Publication Types:
- Review