Prostascint (111In-Capromab Pendetide) (CYT-356)
Clinical problem:
Prostate cancer is the most frequently diagnosed malignancy and second leading cause of cancer deaths in men [2]. The life probability for development of prostate cancer in men in the United States is 1 in 6 [8]. Radical prostatectomy is a highly effective treatment for organ localized prostate cancer [9]. In patients with newly diagnosed prostate cancer, the identification of metastatic disease represents a contraindication to definitive local therapy [8]. Unfortunately, between one-third and half of the patients with suspected clinically localized disease, are found to have extraprostatic disease at prostatectomy [2,5,6]. Metastatic spread of prostate cancer occurs by both hematogenous and lymphatic routes (especially along the pelvic and abdominal great vessels). Patients with nodal or systemic metastases are candidates for palliative rather than curative treatment. Pelvic lymph node dissection is the most accurate method for staging pelvic lymph nodes. However, pelvic lymph node dissection may not correctly identify all patients with metastases [5]. Incomplete sampling can lead to false negative interpretations in 12-33% of patients [5]. Additionally, between 15-50% of pelvic node negative patients may still have para-aortic lymph node metastasis ("skip" metastases) [2,4,6]. External iliac, presciatic, and presacral nodes are also outside the boundaries of the standard PLND [5]. Up to 17% of patients have been reported to have a solitary metastasis in the iliac lymphatic chains outside the conventional area for dissection [10]. The 10 year survival for patients with organ confined disease is about 99%, while survival decreases to 68% for patients with micrometastases to pelvic lymph nodes [6]. Mesenteric lymph node metastases are rare in patients presenting with prostate cancer (0.5%) and are generally found in association with a serum PSA level greater than 50 ng/L and coexistent pelvic lymphadenopathy [12].
The ability to pre-operatively identify patients with non-organ confined disease would greatly affect patient management. Patients with disease not confined to the prostate would be spared futile and potentially morbid surgical intervention or ineffective localized radiotherapy [6]. Independent predictors for the likelihood of extra-prostatic disease include the Gleason Score, the clinical stage, and PSA level. Partin has retrospectively analyzed the probabilities associated with combinations of these three factors, which are determined prior to prostatectomy, to calculate the probability of organ confined disease, the presence of capsular penetration, seminal vesicle invasion, and lymph node metastasis (Partin Tables). These four clinical situations significantly affect patient management.
It has long been recognized that clinical understaging frequently occurs in prostate cancer. Traditional imaging modalities such as CT and MRI rely on anatomic size measurements to identify pathologic lymph nodes. These modalities perform very poorly (sensitivity 4 to 45%) in detecting lymph node metastasis [3,5]. However, both CT and MR have some value in detecting direct extraprostatic extension and bulky nodal disease. Bone scanning, although having a high positive predictive value, is insensitive for metastasis and is used only in those patients with a reasonable probability of metastatic disease (e.g. PSA > 10). ProstaScint is a monoclonal antibody agent that has been used for the evaluation of patients with prostate cancer.
Chemistry and Pharmacology:
ProstaScint is an IgG1 murine monoclonal antibody (Mab 7E11-C5.3) that is produced from the human prostate carcinoma cell line LNCaP [2]. This Mab is directed against a glycoprotein known as prostate specific membrane antigen (PMSA) [2], but not to secretory glycoproteins such as prostate specific antigen (PSA) or prostate acid phosphatase (PAP) [2]. PSMA is more highly expressed in malignant prostate cells compared to non-malignant cells [5,9]. PMSA expression is upregulated in late-stage, androgen independent, and metastatic prostate cancer [17]. Overexpression of PMSA in prostate cancer has been associated with an adverse prognosis [14]. The antibody is reactive with more than 95% of prostate adenocarcinomas [8]. The agent is then conjugated to the 111In chelator GYK-DTPA to form the immunoconjugate 111In Capromab pendetide (CYT-356- ProstaScint). There is a high degree of tumor binding to all prostate cancers tested in vitro, and a very high degree of specificity. There is no significant binding to other cancers or normal tissues, with the exception of mild binding to benign prostatic hypertrophic and normal prostate tissue. Reliable imaging of tumors of a size 0.5 gm and above can be obtained with high quality imaging equipment and careful attention to imaging protocol. This is because ProstaScint uptake is dependent on the degree of PSMA expression, rather than the actual size of a metastatic lesion [6]. PMSA may be expressed by a small number of non-prostatic malignant tumors (renal cell carcinoma and small cell carcinoma of the lung) and these can result in false-positive exams [8].
Approximately 8 +/- 3% of the agent is excreted in the urine within 72 hours. Clearance of the agent via the bowel is also observed [8].
Prostascint is prepared by adding 5 mCi buffered 111In Chloride, then sodium acetate, to 0.5mg capromab pendetide, and filtering this immunoconjugate through a .22um filter, then performing ITLC to assure radiochemical purity. This can be done locally without special equipment, or through a central pharmacy.
Imaging Protocol
A major goal is to distinguish accumulation in common sites of recurrence and metastasis (obturator, internal iliac and, para-aortic lymph nodes, and prostate bed) from adjacent sites of blood pool activity (prostate bed, aortic, inferior vena cava, internal iliac vessels, penis, and bone marrow), urinary excretory structures (kidney, ureter, and bladder), and colonic excretion (large bowel and rectum). To this end it is mandatory that high count, high quality SPECT images be obtained of the pelvis and abdomen at a time when blood pool, urinary excretion and bowel activity are at a minimum. It is very difficult to obtain these images with only a single headed SPECT camera.
A major advantage of Prostascint is that it allows one to survey the entire body, thus permitting the detection of occult metastasis (a second major goal) which can have a major impact on tumor staging. This is best done in conjunction with the late SPECT images to allow background activity to be minimized.
- Obtain a history to include surgical, hormonal, and radiation treatments, serum marker chronology, results of other imaging modalities, clinical and pathologic stage, Gleason sum, and prior history of murine antibody infusion. Sensitivity of the study may be adversely affected by antitumor therapy if the study is being done for staging prior to definitive therapy, and antitumor therapy should be avoided prior to such staging images. Sensitivity may also be affected by prior monoclonal antibody imaging because of altered biologic distribution.
- Infuse Prostascint over 5 minutes and measure vital signs at 10 and 25 minutes after end of infusion. Although rare, hypersensitivity reaction may occur, and appropriate medications and equipment to treat these reactions should be available. There is an approximately 5% incidence of minor reactions.
- Images are usually preformed 96-120 hours (4 to 5 days) following injection to permit a reduction in residual blood pool activity which may obscure uptake in positive nodes [8,9]. Delaying imaging too long decreases count density to a point where a positive node may not be seen [8,9].
- Give the patient a laxative and instruct him to take it the day before the imaging session to clear the bowel of any fecal radioactivity [8].
- An indwelling bladder catheterization, preferably with irrigation, is mandatory to properly evaluate the prostate fossa.
- Obtain skull to mid femur anterior and posterior 8 spot images or equivalent whole body pass imaging.
- SPECT abdomen and pelvis images should be performed using a 128 x 128 matrix based on a 360 degree data set using 3-degree stops and 60 seconds per stop [8]. Reconstruction of backprojected data can be performed using a variety of filters until a clear and satifactory image is obtained [8]. SPECT image reconstruction whould be as close to 1-cm slice thickness as possible. Because the SPECT exam is contains low counts, the backprojected images can be degraded by noise and artifacts [8]. Iterative reconstruction programs can be used to create a less noisy and more accurate image [8]. Additional information can also be obtained at the ProstaScint website: www.ProstaSite.com
- A simultaneous SPECT acquisition of blood pool activity (dual isotope exam) should be acquired using Tc-99m-labelled RBC's 20 to 30 minutes after infusion [8,9]. This permits perfect registration of the data sets and the ability to perform subtraction images [8]. Subtraction images can increase lesion detection rate by up to 62% and can reveal lesions in patients previously felt to be disease free [8].
Interpretation
ProstaScint imaging and exam interpretation is challenging [2]. Good image interpretation requires a knowledge if pelvic and abdominal anatomy, expected sites of metastasis, and more experience in interpretation than is usually expected for simpler procedures. In other words, the learning curve is fairly steep. Excellent imaging technique and processing are prerequisites for adequate interpretation. Side by side interpretation of immediate and delayed images in identical slice sequence is invaluable.
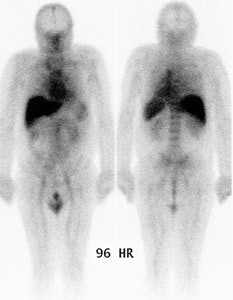
Normal planar prostascint exam: Whole body planar imaging with normal biodistribution of the agent on 96 hour post injection imaging. |
|
- Planar images are first viewed to evaluate extra-abdominal and extra-pelvic structures. Mild blood pool and moderate bone marrow activity, and good visualization of blood filled structures (liver, spleen, penis) is expected. Mild to moderate large bowel activity is frequently seen, and may require bowel recleansing and further delayed imaging. Abnormal mesenteric and abdominal lymph nodes are often better seen on planar than SPECT images. Planar images are almost useless for prostate bed recurrence and pelvic metastasis.
- On SPECT images, identification of pelvic lymph node metastases is largely based on the identifying significant asymmetry from side to side by comparison of the intensity of uptake in the vicinity of the iliac vessels [9].
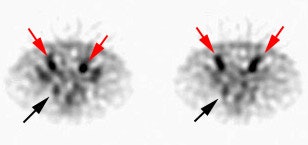
- Carefully evaluate the prostate bed. Asymmetry of activity on delayed images is abnormal in patients who have not undergone prostatectomy. Mild homogeneous, symmetric blood pool activity on early and late images in prostatectomy and RT patients is normal, whereas even minor asymmetric, focal activity is not. Carefully locate the prostate bed in relationship to the bladder and penis on early images and do not be fooled by rectal and pubic ramus activity.
- Don't call lesions at low, e.g. flank near ascending colon
- False positives
- Asymmetric ischial activity- mimics obturator nodes
- Stool in rectum or colon
- Focal inflammatory processes (pneumonia, etc...)
- Residual blood pool activity in vascular structures at the base of the penis [9]
- Asymmetric bone marrow distribution- carefully check early and late images for lack of relative change
- SPECT artifacts (flood inhomogeneities, noisy images)
- Abdominal hernias, bladder and colon diverticulae- accumulate stool and urine
- Fracture sites, Paget's disease, and areas of inflammation (arthritis, bursitis, or tendonitis) [9]
- Adrenal adenoma [9]
Indications for the Prostascint exam:
1- Preoperative Staging in patients with newly diagnosed prostate cancer and a moderate to high probability of extraprostatic metastasis:
Radioimmunoscintigraphy with ProstaScint can enhance staging accuracy in certain patients with newly diagnosed prostate cancer [2]. Studies have focused on the evaluation of patients with a high likelihood for extraprostatic disease based upon an elevated PSA (greater than 40 ng/mL), a high Gleason score (greater than or equal to 7 [with a PSA of at least 20 ng/mL]), more advance clinical T-stage (T2 or T3), or some combination of these criteria [2,5,6]. As such, the patient populations studied have had a high incidence of extra-prostatic disease (39% to 42%) [2,5,6,10]. In this subgroup of patients, extraprostatic disease can be detected with a 62-75% sensitivity, 72-86% specificity, 62-79% positive predictive value, 72% negative predictive value, and 81% accuracy (compared with 4-20% sensitivity, 68% specificity, 48% accuracy, and 31% positive predictive value for CT and MRI) [2,5,6,9,10]. The high incidence of disease within the study groups likely contributes to increased sensitivity; none-the-less, conventional imaging still appears to perform poorly. Also- the sensitivity and specificity for the detection of pelvic lymph node metastatses increases with the number of exams performed [8]. For patients with abnormalities on ProstaScint imaging, it would be prudent for the patient to undergo confirmatory procedures to document metastatic disease such as CT-guided biopsy, abbreviated PLND, or minilaparotomy [5]. False negative exams occur with pathologic lymph nodes that contain microscopic or small tumor volumes (under 5 mm) [2]. As a consequence, a negative scan does not eliminate the need for a staging lympadenectomy [8]. False positive exams have also been described [2].
The primary tumor in the prostate is identified in 88% to 92% of cases- false negative exams are associated with tumors that fail to express the PMSA surface glycoprotein [2,5].
Sites of bone metastases can also be identified, but with decreased sensitivity compared to bone scan [2] (i.e.: bone scan is generally more sensitive than ProstaScint for the detection of bone metastases [9]). Bone scan findings of metastasis are reported to be extremely low in patients with PSA levels less than 20 ng/mL [10]. Currently, the bone scan is reserved for patients with PSA values greater than 10 ng/mL, high Gleason scores (8-10), or those with elevated serum alkaline phosphatase [10].
2- Recurrent Prostate Cancer: Staging in Post-prostatectomy/radiation therapy patients with suspected residual or recurrent prostate cancer (i.e.: rising PSA).
The risk for recurrence after radical prostatectomy correlates with the preoperative PSA, pathologic tumor stage, Gleason score, and positive tumor margins [15]. About 50% of patients with a preoperative PSA of greater than 10 and 70% of patients with a Gleason score of 8-10 have recurrence within 7 years [15]. About 25% of patients with positive tumor margins have recurrence within 5 years [15]. Follwoing radical prostatectomy the serum PSA level should fall to undetectable levels (less than 0.1 ng/mL) within 3-4 weeks [16]. Any detectable PSA level after radical prostatectomy suggests recurrence (PSA remains detectable following radiation treatment- in general recurrence is suspected when 3 consecutive increases in PSA are observed) [15]. The definition of biochemical recurrence varies in the literature from a cutoff value of 0.2-0.5 ng/mL for a single measurement or two consecutive measurements exceeding 0.2 or 0.4 ng/mL [16]. A PSA level of 0.4 ng/mL or greater is strongly associated with disease progression [16]. After radiation, PSA levels decrease slowly, but may never reach undetectable levels [16]. IN these patients, a recurrence is indictaed when there has been an increased in PSA of 2 ng/mL or greater above the patients nadir [16].
Following radical prostatectomy, patients with an elevated or increasing PSA may be candidates for pelvic radiation therapy [11]. This group of patients would include those with any other sign suggesting recurrence [8]. Also included would be patients who had positive surgical margins and who are candidates for adjunct radiotherapy [8]. The intent of local radiation therapy is to eliminate tumor in the pelvis and prostatic fossa and to prolong survival [11]. To achieve this goal, the recurrent or residual tumor must be confined to the radiation field (generally the prostate bed and immediately adjacent area) [11]. Unfortunately, there has been no accurate way to determine if the cancer is confined to the prostatic fossa or whether it has already spread outside the pelvis (an incurable subset of patients) [11]. A prostatic fossa biopsy, even if positive, does not preclude the possibility of distant metastases [9]. In fact, only 25% of men with isolated biochemical evidence of disease after surgery are found to have local recurrence in the absence of distant metastases [9]. ProstaScint offers the potential to identify all areas of residual disease in these cases and identify a subset of patients that will most benefit from local radiation therapy [9]. In this context, the results of the Prostascint exam can have a significant effect radiation therapy recommendations. The exam findings can result in a change in the planned treatment volume in up to 11% of patients [13]. More importantly, by identifying unsuspected sites of disseminated disease, radiation therapy may be withheld in up to 7% of patients [13].
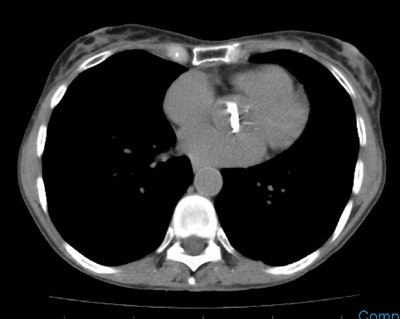
Recurrent/metastatic prostate cancer: The patient below had a history of prostate cancer and a rising PSA level. A lymph node was identified in the posterior right pelvis on CT imaging (white arrow) and described as "borderline" abnormal by size criteria. A Prostascint examination was performed to further evaluate the finding. The Prostascint sudy demonstrated abnormal tracer uptake within the right pelvic node (black arrows), but no other sites of disease (red arrows indicate normal intravascular activity). Percutaneous biopsy of the node confirmed metastatic prostate cancer. |
|
Prostate fossa recurrence can be detected using ProstaScint with a 94% sensitivity, 65% specificity, compared to 27% and 67% respectively for MRI/PET. Specificity may be higher because of fossa sampling error. Sixty percent of patients also had scintigraphic evidence of metastasis outside the prostate bed, but confirmatory evidence is lacking due to the inability to obtain conclusive proof of metastasis in these patients. However, when abnormally increased radiotracer activity is seen outside the prostate bed, a durable complete PSA response following salvage radiotherapy is unlikely [11]. Patients with negative ProstaScint exams or with disease limited to the prostate bed are more likely to have a durable response [11].
Following radiation therapy, intense capromab pendetide localization can persist for years in the rectal, perirectal, and periprostatic soft tissues [9]. This nonspecific uptake is probably related to chronic inflammation and increased capillary permeability as a result of radiation therapy [9]. In patients that have received radiation therapy, ProstaScint is more useful for identifying metastatic disease than for diagnosing persistent or recurrent disease in the prostate bed [9].
Dosimetry
Dosimetry is comparable to other In labeled monoclonal antibodies. Effective dose equivalent is 50 mSv, critical organ is the liver (185 mGy) for a 5 mCi (185MBq) dose [7].
Complications/Side Effects:
Side effects are seen in less than 4% of patients and are generally minor and transient, such as fever/chills (2%), rash/pruritis, hypotension, and headache. Infrequent reactions include chest pain, angioedema, or temporary joint pain. Potentially serious reactions include: Anaphylaxis (although not seen in clinical trials), serum sickness with fever, urticaria, arthralgias, or renal dysfunction. Repeat injection of the tracer (in HAMA negative patients) is associated with a similar adverse reaction rate (2 to 4%).
Caution should be exercised in patients who have previously received murine-based antibody products. Care should also be taken in patients with COPD who may be at a slightly increased risk of bronchospasm.
HAMA Formation (Immunogenicity)
Administration of Prostascint can result in the formation of human anti-murine antibodies (HAMA), which are found in 5-8% [10] (compared to 40% for Oncoscint) of patients after the examination. HAMA levels usually become negative in a few months. No correlation between the presence of HAMA and adverse reactions has been demonstrated [10]. Repeat infusion of the agent can result in elevated HAMA titers in up to 19% of patients, but this is also transient [10]. The presence of HAMA may falsely elevate levels of PSA on in-vitro immunoassays. Special manipulations may need to be performed in these rare instances to compensate for the HAMA influence, and the lab should be notified if this is suspected.
In HAMA positive patients there may be increased concentration of the agent in the liver with diminished blood pool activity.
Conclusion:
Prior to radical prostatectomy, ProstaScint imaging can provide additional useful information that can alter the patient's clinical management [9]. The exam is best reserved for patients at high risk for extraprostatic disease [9]. Due to the low sensitivity for small-volume disease, a negative scan may not eliminate the need for a staging lymph node dissection, but it should encourage further consideration of local treatment options [9]. The agent should be used with caution in patients at low risk of metastatic disease [9]. Positive scans findings in low risk patients should be confirmed prior to any change in treatment (some false-positive scans should be anticipated in a populaiton with a low disease prevalence) [9]. In patients with recurrent disease following primary therapy, ProstaScint can identify metastatic disease in the high-risk patient with a negative bone scan who might otherwise be a candidtae for local salvage therapy [9]. Prostate fossa recurrence may be more difficult to confirm, especially in patients that have received prior radiation therapy [9].
REFERENCES:
(1) Urology 2000; Sodee DB, et al. Multicenter ProstaScint imaging findings in 2154 patients with prostate cancer. 56: 988-993
(2) Cancer 1998; Hinkle GH, et al. Multicenter radioimmunoscintigraphic evaluation of patients with prostate carcinoma using indium-111 capromab pendetide. 83: 739-47
(3) Semin Urol 1995; Burgers JK, et al. Monoclonal antibody imaging of recurrent and metastatic prostate cancer. 13: 103-112
(4) Cancer 1990; Saitoh H, et al. Two different lymph node metastatic patterns of a prostatic cancer. 165:1843-1846
(5) Urology 1999; Manyak MJ, et al. Immunoscintigraphy with indium-111capromab pendetide: Evaluation before definitive therapy in patients with prostate cancer. 54: 1058-63
(6) Cancer 1999; Polascik TJ, et al. Comparison of clinical staging algorithms and 111 Indium-capromab pendetide immunoscintigraphy in the prediction of lymph node involvement in high risk prostate carcinoma patients. 85: 1586-1592
(7) J Nucl Med 1996; Mardirossian G, et al. Radiation absorbed dose from indium-111-CYT-356. 37:1583-8
(8) Nuclear Medicine Annual 2001; Blend MJ, Sodee DB. ProstaScint: An update. Ed. Freeman LM. Lippincott Williams & Wilkins, Philadelphia. 109-138
(9) Techniques in Urology 2001; Rosenthal SA, et al. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. 7 (1): 27-37
(10) Cancer Control 1998; Mayak MJ. Clinical applications of radioimmunoscintigraphy with prostate-specific antibodies for prostate cancer. 5: 493-499
(11) J Clin Oncol 1998; Kahn D, et al. Radioimmunscintigraphy with In-111-labeled capromab pendetide predicts prostate cancer response to salvage radiotherapy after failed radical prostatectomy. 16: 284-289
(12) AJR 2002; Coakley FV, et al. Mesenteric adenopathy in patients with prostate cancer: frequency and etiology. 178: 125-127
(13) J Nucl Med 2004; Jani AB, et al. Influence of radioimmunoscintigraphy on postprostatectomy radiotherapy treatment decision making. 45: 571-578
(14) Clin Cancer Res 2003; Ross JS, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. 9: 6357-6362
(15) AJR 2007; Kundra V, et al. Imaging in oncology from the university of Texas M.D. Anderson cancer center: DIagnosis, staging, and surveillance of prostate cancer. 189: 830-844
(16) AJR 2009; Kelloff GJ, et al. Challenges in clinical prostate cancer: role of imaging. 192: 1455-1470
(17) J Nucl Med 2009; Lapi SE, et al. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. 50: 2042-2048