Renal Vascular Hypertension
Clinical Presentation
Renal vascular hypertension (RVH) is believed to be the etiology of hypertension (HTN) in only 1 to 4% of hypertensive patients. It is the cause of about 5% of all cases of childhood hypertension. A slowly-developing renal artery stenosis allows the opening of collaterals and may result in a severely ischemic kidney with poor function that survives on this collateral flow, but produces large quantities of renin to sustain renovascular hypertension. An acute renal artery obstruction usually leads to partial or complete infarction and atrophy, without hypersecretion of renin. Risk factors for renovascular hypertension include: a diastolic blood pressure greater than 95 mm Hg in a patient that is refractory to 3 antihypertensive medications, accelerated hypertension, sudden loss of previous hypertension control, impairment in renal function following captopril administration, or an abdominal bruit.
A normal renal vein renin level is usually less than about 0.24. A level greater than 0.48 is considered consistent with renovascular HTN (Sensitivity 56-72%, Specificity 30-90%). Unfortunately, up to 65% of patients with non-lateralizing renal vein renin levels will demonstrate improved HTN control after revascularization.
Hemodynamic Significance
Renal Artery Stenosis is considered hemodynamically significant where there is:
- Renin ratio of the affected to the unaffected side of greater than 1.5:1
- Greater than 50% diameter stenosis of the renal artery with post-stenotic dilatation.
- Pressure gradient across stenosis of over 40 mm Hg
As with renal vein renins, specificity in predicting response to therapy is poor.
Etiology of Renovascular Hypertension
Common causes of unilateral or bilateral main or branch renal artery stenosis include:
- Atherosclerosis: Atherosclerotic renal artery stenosis tends to involve the origin (proximal 2 cm) and major bifurcations of the renal artery (although medium sized vessel disease within the kidney may be seen). The involvement may be segmental. ASPVD accounts for 2/3 of the cases of RVH in patients over the 5th decade. Stenosis of the renal artery tends to be progressive and will eventually lead to occlusion of the vessel.
- Fibromuscular Dysplasia: PTCA has an initial success rate of 90% in these patients and a 75% patency rate after 2 years.
- Arteritis: Polyarteritis Nodosa, Takayasu's Arteritis, XRT, or Syphilitic Arteritis
- Aortic Dissection: A dissection of the descending aorta will typically affect the artery to the left kidney.
- Neurofibromatosis: Patients develop a renal artery stenosis due to an underlying mesodermal dysplasia
- Trauma
- Umbilical Artery Catheterization: The placement of an umbilical artery catheter may be complicated by aortic thrombosis, which can produce renal ischemia and RVH due to impingement of the thrombus on the renal artery ostium. Therapy with angiotensin converting enzyme inhibition is generally successful in these neonates, but may precipitate renal failure if the ischemia is bilateral or if there is only one functioning kidney.
- Extrinsic compression of the renal artery or branches
- Renal infarction (total or partial)
- Renal artery aneurysm
- Tumors of the juxtaglomerular apparatus
- Hydronephrosis (Very rarely)
- Other renal abnormalities: Infarction, post-pyelonephritis cortical scarring, and post-traumatic injuries may also result in hypertension.
Pathophysiology of Renovascular Hypertension
In renal artery stenosis, transcapillary pressures to drive glomerular filtration are maintained by a preferential increase in the efferent arteriolar resistance behind the glomerulus. This increased efferent arteriolar resistance is maintained by angiotensin-II (which is produced in response to increased secretion of renin from the involved kidney). Angiotensin II also stimulates aldosterone secretion from the adrenal cortex which contributes to sodium and fluid retention. When a critical level of renal artery stenosis is reached (about 60-70% of the lumen) baroreceptors in the kidney sense the decreased blood pressure in the afferent arteriole, which results in increased renin release from the juxtaglomerular apparatus. This results in increased production of angiotensin I. Angiotensin I is converted in the periphery and the kidney by the action of angiotensin converting enzyme into angiotensin II.
Captopril, an angiotensin converting enzyme inhibitor, acts to block the conversion of angiotensin I to angiotensin-II, which induces efferent arteriole vasodilatation. Thus, post-capillary resistance is decreased and the filtration pressure and GFR fall if the kidney has significant renal artery stenosis. Despite this decline in GFR, effective renal plasma flow does not change significantly. Since the tubular cells retain their functional capacity the kidney will continue to accumulate tubular agents such as MAG3. However, because of insufficient urine production, the tracer is not cleared and remains in the renal cortex. This leads to a prolonged parenchymal transit of the renal tubular tracers.
In patients with a solitary kidney or bilateral critical RAS/RVH, captopril may precipitate acute renal failure, but this effect is usually only transient.
Afferent arteriolar tone is predominantly regulated by calcium entry into smooth muscle cells and may be inhibited by calcium channel blockers (nifedipine and verapamil). Patients taking calcium antagonists may have false positive exams characterized by bilateral symmetric decreased renal function [1].
Treatment of RVH due to Renal Artery Stenosis (RAS)
Percutaneous Transluminal Angioplasty in the Treatment of RAS:
- Atherosclerosis: Following percutaneous translumenal angioplasty for atherosclerotic renal artery stensosis, blood pressure returns to normal in about 10% of patients, improves in 50%, and does not change in 40%. The treatment has a higher success rate (cured or improved) in patients with non-osteal stenoses (88%) than for osteal stenoses (40%).
- Fibromuscular Dysplasia: Blood pressure returned to normal in 60%, improved in 20%, and did not change in 20% after PTCA. Grouping PTCA for both FMD and ASPVD Losinno et al. found a technical and clinical success rate of approximately 80% at 10 years post-procedure. Renal function improved (as evidenced by an improvement in creatinine) in 50% of patients with bilateral stenoses or stenosis to a single functioning kidney following PTCA. [2]
Radiographic Findings in Renal Artery Stenosis
On IVP the affected kidney is usually smooth and small (greater than 2cm size difference from the opposite kidney). RAS is one of the causes of global tissue loss with a smooth contour. There is an initially decreased nephrogram which becomes increasingly dense over time with delayed washout and delayed opacification of the collecting system. Pelvic/ureteral notching may be identified due to the presence of dilated collateral vessels. Arterial calcification may be seen on the scout film.
Angiography can demonstrate the presence of RAS, but gives no physiologic information regarding presence of RVH. Findings include a delayed nephrogram and a stenosis with post-stenotic dilatation. Renal vein sampling can detect the increased renin levels which localize to the involved side in the setting of renovascular hypertension.
Nuclear Medicine Examination of Renovascular Hypertension
Recently, two consensus statements have been published concerning ACE Inhibitor Scintigraphy: the SNM Nuclear Medicine Procedure Guidelines for ACE Inhibitor Scintigraphy (Jan 1996), and the Nineth International Symposium on Radionuclides in Nephrourology Consensus Group Report on ACE Inhibitor Rengraphy [3]. These are remarkably concordant, and are essential reading for anyone performing the examination. Particular attention should be paid to interpretive criteria, especially in the latter, causes of false positive and negative studies, and the criteria for and significance of intermediate problability studies.
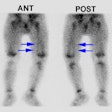
Patients are scanned using Tc-MAG3 before and after the administration of captopril (an angiotensin converting enzyme inhibitor). A positive ACE inhibition scintigraphy exam indicates that RVH is present and implies the existence of hemodynamically significant renal artery stenosis (greater than 60 to 75% of the lumen). For renal artery stenosis > 50% using Tc-MAG3: [4] Sensitivity: 90%, Specificity: 91%, Positive predictive value: 70%, Negative predictive value: 97%. The positive predictive value for clinical improvement in hypertension following revascularization varies between 51% to 100% (mean 85%) [13].
Examination Techniques
Medications can affect the accuracy of the examination. The sensitivity has been shown to decrease from 98% in patients on no medications, to 87% when they are on a diuretic, and 75% if the patient is on an ACE inhibitor [5]. Diuretics should be with-held for the 48 hours prior to the exam. Patients taking calcium antagonists may have false positive exams characterized by bilateral symmetric decreased renal function- these agents should be discontinued prior to the exam [6]. All other antihypertensive medications should be withheld overnight.
Prior to their baseline exam, patients should be off ACE inhibitors for at least 48 hours (generally for 2 to 5 days), and some centers discontinue these medications for up to 7 days [7].
Either Captopril or Enalaprilat can be used as the ACE inhibitor. Captopril 25-50 mg (crushed) p.o. 1 hour prior to start of exam, or Enalaprilat .04mg/kg (Maximum 2.5 mg) via slow (over 5 minutes) I.V. infusion 10-15 minutes prior to the start of the exam. Enalaprilat has a longer duration of action than captopril. The advantage of enalaprilat is that it is administered intravenously and its effect is not affected by a variable rate of absorption through the GI tract. The dose in children is 1 mg/kg up to 50 mg [per Dr. Madj]. BP is monitored every 15 minutes after oral Captopril, or every 5 minutes after I.V. Enalaprilat. It is advisable NOT to proceed with the examination when an adult patient on therapy has a resting systolic pressure less than 140 mm Hg or when an infant has a mean arterial pressure below 65 mm Hg. In these patients, ACE inhibition may result in marked hypotension. If blood pressure falls below safe levels (about 30% of the original systolic value), the rate of I.V. fluid infusion should be increased. Patients should not be permitted to leave the department unless their blood pressure is at least 70% of baseline and they are asymptomatic.
Diuresis: Furosemide 40mg (1mg/kg) may be given at the start of the exam, but is not mandatory. Due to the peripheral action of ACE inhibitors, oliguria is not uncommon. This oliguria may result in retention of radiotracer in the collecting system and the associated renal cortex generating a false-positive difference from the baseline exam. A full urinary bladder may also slow clearance or predispose to reflux; bladder catheterization may be needed in selected cases.
Adequate hydration is essential to maintain renal perfusion. Oral hydration with 10 ml/kg is given prior to the start of the exam. An I.V. line is maintained throughout the exam and 500 ml of Normal Saline is infused at 2-4 ml/min.
Scintigraphic Findings
A positive examination confirms the presence of an activated renin-angiotensin mechanism. This is most commonly secondary to RAS, but other disorders such as vasculitis and scleroderma may also activate this system. A normal ACE inhibitor scintigram indicates a low probability (under 10%) for RVH [13]. A small, poorly functioning kidney (under 30% uptake with a time to maximum activity under two minutes that shows no change on the ACE exam and bilateral symmteric abnormalities such as cortical retention of the agent indicate an intermediate probability of RVH [13].
For tubular agents, findings indicative of a high probability of renal vascular hypertension include:
- Split renal function decrease: Unilateral renal function decreases by more than 5% or is displaced outside normal range (44-56%) after Captopril administration. Other authors quote a decrease of greater than 10% in relative uptake as high probability for RVH [13].
- Increase in time to peak activity (of more than 5 minutes after Captopril compared to baseline.) Other authors quote an increase of 2 minutes in time to peak activity or a change in time to peak activity of greater than 40% as high probability for RVH [13].
- Prolonged Cortical Retention (increased parenchymal transit time): Normally, there should be less than 30% of the peak activity retained in the renal parenchyma at 20 minutes (in fact, with MAG3, there is generally less than 20% retained activity at this time). On the captopril exam, if the retained cortical activity at 20 minutes is less than 30%, this virtually excludes RVH and a baseline exam is not necessary. A decrease in residual cortical activity at 20 minutes of at least 15% on the baseline exam compared to the captopril study is considered diagnostic of RVH. Generally, these patients will have between 70-90% RAS. A 5-10% decrease may be considered suspicious and warrants further evaluation. [8,9]
- Rising Baseline Renogram: Cases of severe stenosis (over 95%) have been seen with a rising baseline MAG3 renogram and no further deterioration after ACE inhibition (Nuclear Medicine Annual, 92, p.175). In these patients the renin-angiotensin compensation is no longer adequate to maintain renal perfusion. Other conditions may also produce a rising baseline exam, including ATN, drug toxicity, interstitial or glomerular nephropathies, complete obstruction, and renal vein thrombosis, so specificity of this finding is suboptimal (50-75%).
- Non-visualization of the kidney: This can be seen in complete obstruction of the renal artery without major collaterals.
Diagnostic Criteria (both consensus statements)
(Based upon evaluation of the renogram curve)
- Grade 0 - Normal
- Grade 1 - Mild delay in upslope with maximal activity seen between 6 and 11 minutes, or delay in the excretory phase.
- Grade 2 - Delay in the upslope and Tmax, but an excretory phase is still seen.
- Grade 3 - As above, but without an excretory phase
- Grade 4 - Renal failure with measureable uptake
- Grade 5 - Renal Failure without measureable function.
Positive Exam: Deterioration in grade following captopril administration is deemed high probability of renal artery stenosis, no change in grade (except grade 0) as indeterminate, and improvement in grade as low probability of renal vascular hypertension. Some consider no change in grade for a grade 1 curve as low probability for RVH.
Prognostic Value of the Captopril Renal Scan:
A positive captopril examination is a strong predictor of surgically correctable renovascular hypertension (97%). Approximately 85% to 90% of patients with an abnormal exam will demonstrate improvement in blood pressure following intervention [10,13]. No change in renal function after captopril in a patient with known RAS predicts a poor response to angioplasty or surgery; however, about 30% of these patients will still demonstrate some improvement following intervention.
In cases of severe RAS (greater than 90%) the compensatory functionof the renin-angiotensin system may be incomplete, and the baseline study in these patients can be abnormal. These kidneys may be atrophic and hypofunctioning. A detectable change may not be appreciated in these patients following the administration of captopril.
False positive exams: Patients who develop marked hypotension (Systolic BP < 100 or a blood pressure drop of greater than 20 mmHg) following ACE inhibitor administration may have a false-positive examination. Frequently this effect is bilateral [14]. Patients with low-output congestive heart failure may have false-positive examinations. Renal arteriolar vasculitis may also produce a false-positive exam.
Tc-DTPA (Glomerular Agent) in the Evaluation of RVH
A decrease in the cortical uptake between 2-3 minutes of the examination is expected to occur in RVH. This can be seen as a flattening of the time-activity curve with a prolonged time to peak renal activity (a change of at least 2 minutes to peak activity or over 11 minutes on either the pre- or post-captopril exam), or expressed as a change in the split or absolute renal functions at these times- a unilateral decrease in differential function of at least 5-9% (suspicious) or greater than 10% (high probability). Another criteria is the GFR ratio between the kidneys which will becomegreater than 1.5 on the post-captopril exam. In severe stenosis (over 95%), the affected kidney will be hypofunctioning and a captopril effect may not be seen. A diuretic is not necessary for this examination because RVH criteria are based upon the rate of accumulation of the tracer, NOT on excretion. However, marked unilateral retention, although uncommon, represents a high probability study.
Captopril Scintigraphy in Patients with Hypertension and Chronic Renal Failure
Hypertension is an invariable consequence of the loss of renal function in patients with chronic renal failure. ACE inhibitors are now being used with increasing frequency in patients with hypertension and renal failure since there is evidence to suggest that they may be renoprotective. However, in clinical situations where the renin-angiotensin system is activated, ACE inhibitors can have a detrimental effect on renal function.
In these patients, a normal captopril exam may indicate a beneficial effect of ACE inhibition on renal function. At a minimum it indicates that the danger of treating the patient's hypertension with an ACE inhibitor is minimal. If the study is abnormal, the renin-angiotensin system is activated and ACE inhibition therapy is contraindicated. Once GFR drops below 10 ml/min. or split function becomes 10% or less, Tc-MAG3 captopril scintigraphy in these patients is generally unreliable [7,10]. Other authors feel that Captopril scintigraphy can still adequately detect renal artery stenosis in renal failure patients [12].
REFERENCES:
(1) J Nucl Med 1998; Claveau-Tremblay R, et al. False-positive captopril renography in patients taking calcium antagonists. 39: 1621-1626
(2) AJR 1994; Apr: 853
(3) J Nucl Med 1996; Taylor A, et al. Consensus report on ACE inhibitor renography for detecting renovascular hypertension. Radionuclides in Nephrourology Group. Consensus Group on ACEI Renography. 37(11):1876-82. No abstract available.
(4) Dondi et al, EJNM, 1990
(5) HTN 1991; 18; 289-98
(6) J Nucl Med 1998; Claveau-Tremblay R, et al. False-positive captopril renography in patients taking calcium antagonists. 39: 1621-1626
(7) J Nucl Med 1994; Datseris IE, et al. Captopril renal scintigraphy in patients with hypertension and chronic renal failure. 35(2):251-4.
(8) Radiol Clin North Am 1993; Sfakianakis GN, et al. Diagnosis of renovascular hypertension with ACE inhibition scintigraphy.31(4):831-48. Review. No abstract available.
(9) Nuclear Medicine Annual 1992; p.175
(10) J Nucl Med 1994; Blaufox MD. Should the role of captopril renography extend to the evaluation of chronic renal disease? 35(2):254-6. No abstract available.
(12) J Nucl Med 1999; Fernandez P, et al. Value of captopril renal scinitgraphy in hypertensive patients with renal failure. 40: 412-417
(13) Radiographics 2000; Soulez G, et al. Imaging of renovascular hypertension: Respective values of renal scintigraphy, renal doppler US, and MR angiography. 20: 1355-1368
(14) J Nucl Med 1999; Stavropoulos SW, et al. Hypotensive response to captopril: A potential pitfall of scintigraphic assessment for renal artery stenosis. 40: 406-411