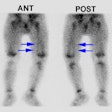
Renal Transplant Evaluation
Renal transplant has become the treatment of choice for end-stage renal disease [1]. One year survival rates are reported to be 80% for mismatched cadaveric renal grafts; 90% for non-identical living related grafts; and 95% for human lymphocyte antigen-identical grafts [1].
Imaging was previously done using Tc-DTPA and 131I Hippuran, but presently most centers are using a single agent- Tc-MAG3.
When evaluating perfusion, the vascular blush of a normal functioning transplant should be almost simultaneous with that of external iliac artery and peak activity should equal that of the iliac artery. Extraction, excretion, and clearance of tracer activity should normally be similar to that of a native kidney.
Transplant Rejection
Clinically, transplant rejection is characterized by oliguria, weight gain, and constitutional symptoms such as fever and pain. Generally, urinary sodium is decreased (as opposed to ATN in which the urinary sodium is often increased). Physiologically, rejection is manifested by decreased perfusion at the small vessel level. There are 3 forms of rejection: Hyperacute, Acute, and Chronic.
1- Hyperacute Renal Transplant Rejection:
Hyperactue rejection often begins within minutes to hours (usually by 24 hrs) of the transplant. It is due to the presence of pre-formed cytotoxic antibodies at time of transplant. It is an irreversible process. Pathologically there are thrombosed arterioles and cortical necrosis. Treatment is immediate reoperation.
2- Acute Renal Transplant Rejection:
Accelerated acute rejection refers to rejection which occurs between 1 to 5 days post transplant and is most commonly seen in patients who have received previous transplants.
Acute rejection typically occurs 1 to 5 weeks following transplant (usually within 3 months). It is primarily a cell-mediated antigen-specific immune response, but antibodies are often found as well. Both accelerated acute and acute rejection are potentially reversible processes. Up to 50% of renal transplant patients experience at least one episode of acute rejection during their first year [1]. Patients typically present with malaise, fever, weight gain, or a painful kidney [1]. Patients receiving cyclosporine may be asymptomatic [1].
On scintigraphy, acute rejection is characterized by decreased renal perfusion and decreased tubular function which progressively worsens on serial evaluation. The nephrogram phase is prolonged and there will be delayed bladder visualization. Increased perinephric accumulation of Tc-DTPA signifies end-stage rejection as manifested by renal infarction.
Tc-Sulfur Colloid has been shown to accumulate within transplanted kidneys that have undergone an episode of rejection. This occurs due to trapping of the labeled particles in small vessels occluded by fibrin thrombi. Transplant uptake will be more intense than that in surrounding bony structures. False-positive Tc-SC exams have been reported with infections and with high dose steroids [Seminars,Jan.95, p. 54-5]. In-111 platelets also accumulate in rejected renal transplants secondary to small vessel occlusion. A kidney-to-background ratio over 1.5 is associated with rejection. Labeled fibrinogen, gallium-67, and In-111 WBC's have also been used in the evaluation of transplant rejection with varying success.
3- Chronic Renal Transplant Rejection:
Chronic rejection can be seen 3 months to many years following transplantation. It is primarily mediated by a cellular immune response. Chronic rejection is a slow, insidious process characterized by gradual, progressive deterioration of allograft function over months to years. The kidney will be small and shrunken. Histologically, there is endothelial proliferation which leads to occlusion of small vessels, basal membrane thickening, and interstitial fibrosis. Although no therapy is available, chronic rejection may stabilize and borderline function can be maintained for years. Chronic rejection is the most common cause of late graft loss [1].
On scintigraphy, chronic rejection is characterized by decreased perfusion, a decreased accumulation of activity (maximum counts or peak activity), normal to slightly delayed time to peak activity (5 to 7 minutes), and a moderately increased cortical retention at 20 minutes (although parenchymal transit and cortical retention may be normal). Bladder activity may appear normally, or be slightly delayed (5 to 8 minutes) [2,3].
Doppler US has not proved to be as accurate for the evaluation of rejection as initially thought [1]. Ultrasound findings in rejection include decreased diastolic flow and an increased resistive index .
(Peak Systolic Vel.- End Diastolic Vel.) RI = ----------------------------------------------- Peak Systolic Vel.
A RI greater than 0.8 is abnormal. A RI of 1.0 indicates absence of diastolic flow, while an RI greater than 1.0 indicates reversed flow during diastole.
Other Transplant Complications
ATN
Generally an early post-op complication (see below on ATN)
Hematoma
A small crescentic peritransplant fluid collection seen immediately after transplantation is most likely a hematoma or seroma [1].
Lymphocele
(15% incidence)
Lymphoceles generally require some time (4 to 8 weeks) before they become large enough to produce symptoms. Patients are usually asymptomatic, but can present secondary to symptoms of obstruction. Lymphoceles are felt to occur secondary to ligation of the normal lymphatic channels at the time of transplant (patients also frequently develop lower extremity edema on the same side as the transplant). The differential considerations include a hematoma or a urinoma, but these usually present sooner.
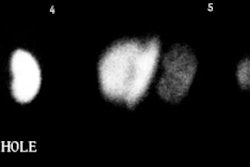
Lymphoceles usually appear as a photodeficient region adjacent to the transplant on scintigraphy. On ultrasound they can have a multiseptated appearance.
Ureteral Obstruction
(1-10% incidence)
Ureteral obstruction can occur due to stricture formation (most commonly at the UVJ anastamosis), a blood clot, edema at the anastamosis, or a lymphocele.
Urine Extravasation/Urinoma
(Rare to 10% incidence)
Urine leaks are associated with a high mortality. Symptoms include urine leaking from the surgical wound, pain, fever, and an increased creatinine. It generally occurs within the first few days post-op and the most common site is at the ureterovesical anastamosis (related to surgical technique). Urine leaks elsewhere in the collecting system usually develop secondary to ischemia with resulting necrosis [1]. On US, internal septations are noted less commonly than in hematomas [1].
Vascular Complications
- Renal Artery Stenosis (1-10%): Renal artery stenosis is the most common vascular complication of renal transplant [1]. Most commonly stenosis occurs at the anastomotic site and is short segment. Patients present with hypertension and worsening renal function. Captopril scintigraphy is useful in these cases [Nuc Med Communications, 14(11)969-75, 1993]. Sonographic findings which suggest stenosis include a flow velocity of greater than 2 m/sec; a velocity gradient between the stenotic and pre-stenotic segments of more than 2:1; and marked spectral broadening (indicative of turbulence) [1].
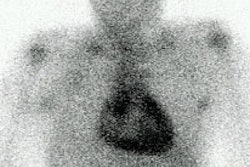
- Renal artery occlusion: Commonly associated with allografts of disparate vessel size or multiple anastamoses. The scintigraphic findings of a photon deficient transplant compared to background indicates the organ is not salvageable.
- Pseudoaneurysm: Usually the result of vascular trauma associated with percutaneous biopsy.
- Renal vein thrombosis: A rare cause of transplant dysfunction which usually occurs within the first week after transplantation [1]. Patients develop sudden oliguria and graft tenderness. There is an increased frequency of renal vein thrombosis in left lower quadrant allografts due to compression of the left common iliac vein between the sacrum and the right common iliac artery [1].
Acute Tubular Necrosis (ATN)
Etiologies of ATN Include:
1- Early post-op renal transplant complication
Typically develops within 24 to 48 hours post-transplant and is rare after 1 month. Presently, transplant patients who require dialysis for the first week post-op due to ATN are said to have "delayed graft function." ATN is more frequent in cadaveric transplants due to ischemic damage prior to grafting and reperfusion injury [1]. Cold ischemia times of more than 24 to 30 hours result in a higher frequency of ATN [1]. ATN is initially present in most cadaveric grafts and resolves spontaneously over the first two weeks. ATN is infrequently seen in patients that receive grafts from living related donors [1].
Other causes of ATN include:
2- Hypotension/Ischemia; 3- Nephrotoxins; 4- Drug toxicity Aminoglycosides; 5- Ethelene glycol, Carbon tetrachloride; 6- IV contrast: Especially in patients with diabetic nephropathy; 7- Heavy metals: Mercury
Clinical Findings in ATN:
The primary symptom of ATN is oliguria. Transplant patients with ATN generally lack the constitutional symptoms which are seen in acute transplant rejection. Also, the renal dysfunction associated with ATN is generally temporary and will improve with time. On lab analysis the urinary sodium is generally elevated due to loss of concentrating ability by the kidney (tubular dysfunction).
Radiographic Findings
On IVP in non-transplant patients, the kidneys are smooth and enlarged. One can see an immediate, persistent, or increasingly dense nephrogram (75%), with little to no collecting system activity.
Scintigraphic Findings
On scintigraphy, perfusion is typically maintained or only slightly reduced, but GFR is very decreased. There is poor but progressive accumulation and delayed excretion of renal tubular agents (due to tubular dysfunction).
In transplant patients the findings of ATN may be indistinguishable from acute transplant rejection. The time course and symptoms are key to the diagnosis. On serial examination, renal transplant dysfunction secondary to ATN should improve or remain unchanged, while that due to rejection demonstrates progressive deterioration.
Differential Diagnosis of ATN in the Renal Transplant Patient:
1- Cyclosporin toxicity
(used to prevent rejection)
Cyclosporin produces a vasoconstrictive effect on the afferent glomerular arterioles [1]. A cyclosporin level greater than 300 ng/ml may suggest toxicity as the cause of poor allograft function. Although cyclosporin toxicity is not typically seen within the first month following transplant, nephrotoxicity can occur within 2 to 3 days of high dose cyclosporin administration. Severe cyclosporin toxicity classically produces findings similar to ATN on scintigraphy- with preserved perfusion, but poor tubular function. Renal perfusion may occasionally be impaired due to a reversible vasoconstriction of the afferent arterioles associated with the initial nephrotoxic effects of the agent. In this setting, the appearance will be similar to acute rejection. Resolution of cyclosporin toxicity with dose adjustment is usually rapid, permitting the retrospective diagnosis. In the later stages, there is irreversible injury to the renal microvasculature and interstitial fibrosis. The scintigraphic findings during this stage may be difficult to distinguish from chronic rejection. Although cyclosporin toxicity is usually a generalized process, focal areas of more prominent dysfunction may be seen. [2,4]
2- Acute rejection
A major point of distinction between rejection and ATN is that perfusion is compromised in rejection, but remains relatively normal in ATN.
3- Systemic hypotension
4- Obstruction
5- Acute renal failure
Drug induced, Urate nephropathy, Renal vein thrombosis
REFERENCES:
(1) Radiographics 2000; Brown ED, et al. Complications of renal transplantation: Evaluation with US and radionuclide imaging. 20: 607-622.
(2) Nuclear Medicine Annual 92, p.195
(3) Semin Nucl Med 1995; Dubovsky EV, et al. Radionuclide evaluation of renal transplants. 25(1):49-59. Review.
(4) Critical Reviews in Diagnostic Imaging 1991; Taylor A. Radionuclide evaluation of renal function. 32(1):1-36