Adrenal Imaging
Meta-iodobenzylguanidine (MIBG)
Pharmacology & Physiology
Structurally, MIBG resembles norepinephrine and guanethidine (a neurosecretory granule depleting agent). MIBG images the adrenal medulla (NOT the adrenal cortex) and sympathetic nervous tissue. It can be labeled to either 123I or 131I, but only I-131 labeled MIBG is available commercially. 123I must be produced locally and this is done at certain academic institutions. If using 123I, a 7 fold larger dose is permitted. This provides a 10 fold higher photon flux at 24 hours, and is still 3 times higher at 48 hours. The 159 keV photon of 123I is also more easily collimated and the images obtained are of higher quality. 123I images have improved spatial resolution, but this is generally not important in "hotspot" imaging and there is no demonstrable difference between the two tracers in detecting neuroblastoma reported in the literature.
MIBG localizes to storage granules in adrenergic tissue of neural crest origin. Its uptake appears to occur through the energy requiring, sodium dependent (active transport) Type 1 amine uptake mechanism and there is some non-energy dependent diffuse (Type II) [12]. Once inside the cell, MIBG is concentrated in the intracellular hormone storage vesicles by an energy dependent, tetrebenazine (reserpine)-sensitive mechanism [1]. Uptake is proportional to the number of neurosecretory granules within the tumor. In neuroblastoma, the agent remains within the cellular cytoplasm, free of granular storage. It's retention in neuroblastoma is related to the rapid re-uptake of agent that has escaped the cell [12].
In childhood, extra-adrenal chromaffin tissue is abundant. The largest mass of such tissue is the Organ of Zckerkandl which is located anteriorly below the origin of the IMA. After the age of 3 years, these extra-adrenal sites regress except in sympathetic ganglia and the carotid bodies.
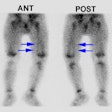
Normal distribution of I-131 MIBG:
- Adrenal medulla
- Liver: Uptake is inversely related to catecholamine levels
- Spleen
- Urinary bladder: Due to renal excretion of the tracer
- Colon: Activity is seen in up to 15-20% of patients
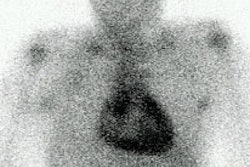
- Salivary glands: Due to rich sympathetic innervation
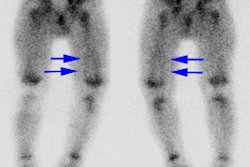
- Heart/myocardium: Uptake is inversely related to catecholamine levels.
- Lung
- Thyroid: May be seen in a small number of patients, even if it is being blocked.
- Uterine and neck muscle uptake [3]
- Upper thorax in children [4]
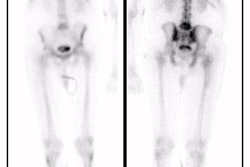
131I: The adrenals are usually not seen, but may be faintly visualized in
10-20% on delayed images. The larger the dose of tracer used, the more likely the normal
adrenal gland will be visualized. Also, due to the improved specific activity of MIBG used
for imaging, increased visualization of the normal adrenal gland has been noted (up to 73%
of patients on delayed imaging) [1]. The intensity of uptake in the normal adrenal should
be less than the liver.
123I: Adrenals are seen in 30% of patients. Although the activity is generally
mild (less than liver intensity) and symmetric, it can be unilateral or asymmetric in up
to 50% of cases. Confusion can then occur in differentiating this uptake from a
pheochromocytoma. Unilateral uptake less than or equal to the liver can represent
pheochromocytoma in 10% and 33% of cases, respectively. Unfortunately, 31% of normal
adrenals are also visualized with uptakes less than or equal to the liver. The absence of
an anatomic lesion on the abnormal side can be helpful in excluding a tumor. In general,
most pheochromocytomas demonstrate uptake more intense than the liver (80%). [2]
Excretion
Most MIBG (about 85-90%) is excreted unchanged in the urine by 96 hours post injection. There is variable excretion into the gut (1 to 4%). The whole body half-life is about 24 hours, but the agent is retained in sympathetic nervous tissue for a prolonged period of time.
Indications
MIBG is used primarily to image Pheochromocytomas (Paragangliomas) and Neuroblastoma. Other APUD cell line tumors-- e.g. carcinoid, and medullary thyroid carcinoma-- demonstrate uptake of the tracer less frequently. Adrenal medullary hyperplasia may also be imaged.
Technique
Dose: 500 uCi 131I or 3 to 10 mCi 123I. Imaging is performed at 1 and 3 days following 131I administration, and 6 and 24 hours after 123I administration. Thyroid uptake of the tracer is blocked using Lugol's solution or SSKI. This should be continued for 5 to 6 days to block uptake of free iodine. The critical organ is the adrenal medulla (100 rad/ mCi 131I).
Drugs which interfere with MIBG uptake
- Cocaine: Inhibits catecholamine uptake [12]
- Tricyclic antidepressants (Desipramine, Amitriptyline [Elavil]): Inhibit catecholamine uptake [12]
- Phenylpropanolamine/Pseudephredine/Phenylephrine- found in over the counter cold preparations and decongestants these agents deplete storage vesicle contents
- Catecholamine agonists: Sympathomimetics, Amphetamines, and Amphetamine-like compounds. Deplete storage vesicle contents [12]
- Reserpine: Depletes the catecholamine stores in the neurosecretory storage granules and inhibits vesicle active transport [12].
- Antipsychotics: Phenothiazines (Thorazine) and thiothixines inhibit catecholamine uptake [12]
- Calcium channel blockers
- Adrenergic blockers: Long acting beta-blockers: Labetalol and possibly metoprolol. Labetalol inhibits catecholamine uptake and depletes storage vesicle contents [12]. It should be discontinued for at least 3 weeks prior to scanning. The absence of salivary and parotid activity can serve as a clue that patients may be on this medication.
- Possibly some foods: Those which contain vanillin and catecholamine-like compounds such as chocolate and blue-veined cheeses
Disease Processes and Neoplasms Imaged with MIBG:
Pheochromocytoma
Clinical Presentation
Pheochromocytomas arise from chromaffin cells (neural crest ectoderm involved with catecholamine synthesis) which is found in the adrenal medulla, sympathetic ganglia, Organ of Zckerkandl, and the aortic and carotid chemoreceptors.
Patients can present with paroxysmal HTN (50%) or sustained HTN (40%) associated with headache, sweating, palpitations, weight loss, and hyperglycemia. Only 0.05% of patients with hypertension are found to have pheochromocytomas.
Laboratory abnormalities include elevated urinary catecholamine, VMA (50%) and metanepinephrine levels.
Pheochromocytomas follow a rule of "ten": 10% are malignant (tend to be larger lesions), 10% are bilateral, 10% occur in pediatric patients, and 10% are extra-adrenal. Glucagon and IV contrast are contraindicated in these patients as they may cause catecholamine release from the tumor and precipitate a hypertensive crisis. Pheochromocytomas are associated with certain disorders such as: Neurofibromatosis, von Hippel-Lindau (develop in 10% of patients), MEA syndromes IIa & IIb (bilateral pheochromocytomas are found in up to 75% of these cases), and Carney's syndrome (which consists of smooth muscle tumors of the stomach, chondromatous pulmonary hamartomas, and extra-adrenal paragangliomas).
Extra-adrenal lesions occur in 10% of adults, and 30% of children. Extra-adrenal lesions are generally located in the retroperitoneal sympathetic ganglia in the paracaval/aortic region or near the organ of Zckerkandl. Other sites include the renal hilum (may have associated ipsilateral renal atrophy), the mediastinum (1%), and the urinary bladder (1%- associated with micturitional syncope). In adults, extra-adrenal lesions are malignant in 30-40% cases, while despite their higher prevalence, only about 2% of extra-adrenal lesions are malignant in children.
Paragangliomatosis refers to the presence of "too numerous to count" pheochromocytomas. This condition is noted in about 5% of patients with extra-adrenal lesions. In this condition, the majority of the lesions are benign, but they may still be functionally active.
Radiographic Findings
On CT most pheochromocytomas are greater than 3 cm in size. They are usually soft tissue density and the center of the lesion may be necrotic. The lesion may appear completely cystic. Calcification is noted infrequently. On MRI, the lesion is usually slightly less intense than the liver on T1 images, but extremely bright on T2 images. Occasionally, lesions may be inhomogeneous, iso-, or hypointense to the liver. Angiography is contraindicated unless absolutely necessary as it may provoke a hypertensive crisis. In such cases, premedication is required. The lesion is typically hypervascular.
Scintigraphic Findings
MIBG scan sensitivity for pheochromocytoma is 86% and specificity is 95-99% [5]. In patients with biochemical abnormalities indicative of a pheochromocytoma, CT or MRI are more accurate in detecting primary tumors of the adrenal gland, but they are inferior to MIBG in the detection of extra-adrenal tumors which account for about 10% of lesions [6]. Unlike neuroblastoma, bone scan is superior to MIBG for detecting the presence of bone metastases in patients with pheochromocytoma. On MRI, pheochromocytomas have a signal intensity similar to or slightly lower than the liver on T1 images, but are extremely hyperintense on T2 images. Unfortunately, due to the presence of necrosis within the lesion, over 20% of lesions will have an atypical appearance on T2 images [5]. Approximately 10% of pheochromocytomas are cystic, but these lesions are still endocrinologically active and will concentrate MIBG.
Neuroblastoma
Clinical Presentation
Neuroblastoma is the most common extracranial solid malignant tumor of infants and children. About 50% of cases occur in children less than 2 years of age, and 90% are seen by age 8. Males are affected slightly more than females. Associations with a familial predisposition (0.2%), bowel agangliosis, and CHD have been described, but none are very strong.
Symptoms of neuroblastoma include an abdominal mass, limp, nystagmus, or inability to walk. Hypertension is found in 30% of patients. Elevated urinary catecholamine levels (VMA & HVA) are seen in 90%. Metasases are present in 60% of patients at the time of diagnosis. The skeleton is the most common site (60%). Metastases can also go to lymph nodes (40%), the liver, and intracranial (dura). Prognosis is related to patient age (younger age [esp. under 1 year]->better prognosis), and tumor grade (lower grade or Stage IVs->better prognosis). Occasionally, the lesions may revert to a benign ganglioneuroma (0.2% of cases).
Paraneoplastic syndromes are also seen and include:
- Infantile myoclonic encephalopathy
associated with occult neuroblastoma in 20-50% of cases, it is the initial presentation of neuroblastoma in about 2% of children. The syndrome consists of opsoclonus (random irregular eye movements or "dancing eyes"), myoclonus,and cerebellar ataxia.- Verner-Morrison syndrome
Watery diarrhea, hypokalemia, and achlorhydria. Seen in 5% of cases, it is secondary to VIP production by the tumor (elevated VIP levels are noted in 25% of patients with neuroblastoma)
Sites of Primary Neuroblastoma
75% of cases are intraabdominal.
Adrenals:
Most frequent (50% of cases) intra-abdominal site. About 10% of cases are bilateral.
Primary adrenal lesions are actually associated with a worse prognosis than extra-adrenal
lesions.
Extra-adrenal abdominal sympathetic ganglia
Includes the Organ of Zckerkandl (a cluster of paraganglia near the aortic bifurcation, seen predominantly in children).
Intrathoracic
Approximately 15% of cases are intrathoracic. The lesion generally arises posteriorly in
the paravertebral sympathetic chain. About 25% will have an associated intraspinal
component. Thoracic and cervical primaries are associated with a better prognosis.
Cervical
Intracranial
Rare (approximately 2% of cases). Most commonly arises in the olfactory nerve (esthesioneuroblastoma), or cerebellum.
Staging of Neuroblastoma
- Stage I: Confined to the organ of origin
- Stage II: Extends into adjacent structures, but does not cross midline. Includes extra-adrenal primaries.
- Stage III: Crosses midline
- Stage IV: Distant metastases
- Stage IVs: Primary lesion does not cross midline, with widespread metastases to liver, skin, and bone marrow (but NOT bone). It is usually seen in infants under 1 year of age. Associated with very good survival following XRT and chemo.
Scintigraphy in the Evaluation of Neuroblastoma
Patient preparation/imaging: A solution of potassium iodide is administered to reduce thyroid uptake of free radioiodine, beginning the day before injection and continued for 5 to 6 days. I-131MIBG (0.5-1.0 mCi/1.7 m2 body surface area [7 to 15 uCi/kg]) is administered by slow IV injection over 90 seconds. Images of the entire body should be obtained. Images are acquired for 100,000 counts or 20 minutes, whichever comes first. A low-speed, whole body gamma camera can also be used to obtain the images. [12]
Findings: The overall sensitivity of MIBG in the detection of neuroblastoma is approximately 90% [7,12]. The overall specificity of MIBG in the detection of neuroblastoma is 94% [12]. The extent of metastatic bone and bone marrow disease is most accurately defined by MIBG scintigraphy. Bone scanning often grossly underestimates the extent of osseous metastases and MIBG is superior to MDP for detecting the presence of skeletal metastases (likely because of the propensity for neuroblastoma metastases to originate within the bone marrow cavity) [8,12]. Normalization of MIBG exams in patients with documented bone marrow metastases is a better predictor of successful treatment than MRI exams which can demonstrate persistent abnormal marrow signal despite the absence of metastases for up to 2 years [9]. Liver metastases may escape detection with MIBG imaging. This finding is not surprising as MIBG is physiologically concentrated in the liver, and therefore small lesions may be obscured [10]. SPECT increases diagnostic certainty, but does not increase lesion detection with I123-MIBG [11].
TcMDP is concentrated in about two-thirds of primary lesions (and is felt to be related to presence of calcification). Bone scan detects the presence of skeletal metastases in 40-60% of patients, but localization of metaphyseal metastases (a common site of metastatic disease) can be difficult due to the adjacent physeal activity. A common finding on bone scan in patients with metaphyseal metastases is ill-defined increased activity in this area with loss of the sharp boundary between the growth plate and the metaphysis.
In-111 Octreotide can also be used to image neuroblastoma, but it is inferior to MIBG as non-neuroendocrine tumors such as lymphoma and inflammatory infiltrates may also demonstrate tracer uptake [12].
Correlative Radiographic Imaging in Neuroblastoma
Plain film findings in neuroblastoma include multicentric lytic skeletal lesions, metaphyseal lucent bands, and vertebral body collapse. On IVP there is typically inferior renal displacement rather than collecting system distortion (seen in Wilm's)
On CT neuroblastoma typically appears as a large complex mass which is often locally invasive into adjacent tissue and typically encases the great vessels, although a pseudocapsule maybe seen. Neuroblastomas often contain areas of necrosis, hemorrhage, and calcification (up to 80%). Calcification can be curvilinear or nodular. The mass may appear cystic and can mimic an adrenal hemorrhage in neonates. Neuroblastomas appear bright on T2-weighted MRI images.
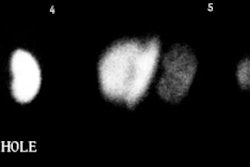
Adrenal Medullary Hyperplasia
In patients with adrenal medullary hyperplasia, the level of accumulation of the
radiotracer is related to the degree of medullary dysfunction. The exam typically reveals
symmetric or nearly symmetric increased tracer uptake within the glands, with relative
preservation of the glandular shape. Since the right adrenal gland is more superficial on
posterior images, it may appear more intense than the left side.
REFERENCES:
(1) J Nucl Med 1998; Roelants V, et al. Iodine-131-MIBG scintigraphy in adults: Interpretation revisited? 39: 1007-1012
(2) J Nucl Med 1994; Mozley PD, et al. The efficacy of iodine-123-MIBG as a screening
test for pheochromocytoma.
35: 1138-1144
(3) Clin Nucl Med 1995; Elgazzar AH, et al. I-123 MIBG scintigraphy in adults. A report of clinical experience. 20(2): 147-152
(4) J Nucl Med 1994; Bonnin F, et al. Refining interpretation of MIBG scans in children. 35(5):803-10
(5) Semin Nucl Med 1995; Freitas JE. Adrenal cortical and medullary imaging. 25(3):235-50
(6) J Nucl Med 1993; Maurea S, et al. Iodine-131-metaiodobenzylguanidine scintigraphy in preoperative and postoperative evaluation of paragangliomas: comparison with CT and MRI. 34: 173-79
(7) Semin Nucl Med 1993; Gelfand MJ. Meta-iodobenzylguanidine in children. 23(3):231-42
(8) J Nucl Med 1993; Parisi MT, et al. Optimized diagnostic strategy for neuroblastoma in opsoclonus-myoclonus. 34(11):1922-6
(9) J Nucl Med 1997; Lebtahi N, et al. Evaluating bone marrow metastasis of neuroblastoma with Iodine-123-MIBG scintigraphy and MRI. 38: 1389-1392
(10) J Nucl Med 1994; Maurea S, et al. Iodine-131-MIBG imaging to monitor chemotherapy response in advanced neuroblastoma: comparison with laboratory analysis.35(9):1429-35.
(11) J Nucl Med 1994; Gelfand MJ, et al. Iodine-123-MIBG SPECT versus planar imaging in children with neural crest tumors. 35(11):1753-7
(12) J Nucl Med 1998; Shulkin BL, et al. Current concepts on the diagnostic use of MIBG in children. 39: 679-688