Pyelonephritis and Renal Scarring
Clinical Presentation
Pyelonephritis is generally the result of an ascending infection. Less commonly it can occur secondary to hematogenous dissemination. Vesicoureteral reflux was once thought to be a necessary prerequisite for the development of pyelonephritis and subsequent renal scarring; however, reflux is seen in only 30-50% of affected children. Therefore, about 50% of the cases of pyelonephritis occur in the absence of reflux and bacterial virulence factors may play a role in these cases. Infections by certain strains of E.coli which release O antigen may paralyze the ureter causing a functional obstruction. Other strains of E.coli have fimbriae which provide them with mobility. Higher grades of reflux are a risk factor for pyelonephritis, but in patients with mild reflux, their risk for pyelonephritis is the same as in those without reflux.
The younger the child, the more nonspecific the signs of pyelonephritis are [11]. Infants with pyelonephritis typically present with fever, lethargy, irritability, and failure to thrive. Toddlers may present with fever, abdominal pain, vomiting, diarrhea, and feeding problems. Older children are more likely to have the typical adult symptoms of fever, chills, and flank pain. Symptoms of a lower urinary tract infection (frequency, dysuria, and urgency) may also be present. E. coli, Proteus, and Klebsiella are the common inciting organisms.
Pathophysiology of Pyelonephritis
Pathophysiologic changes in acute pyelonephritis include tubulo-interstitial inflammation/pus with impairment of the renal microcirculation due to compression of the glomeruli, small peritubular capillaries, and vasa rectae by interstitial edema. The resultant ischemia has been postulated as one of the mechanisms for the decreased accumulation of DMSA in areas of pyelonephritis. Later there is the formation of micro-abscesses and suppurative necrosis.
Most cases of acute pyelonephritis resolve without scarring. Pyelonephritis occuring before a child is 2-3 years old is believed to reult in the highest risk for subsequent renal scarring [1]. In patients with renal scarring, scars are identified only at sites corresponding to previous areas of acute pyelonephritis. Once acute pyelonephritis has occurred, ultimate renal scarring is independent of the presence or absence of vesicoureteral reflux (Scarring is identified equally in patients with and without reflux). Patients with bladder pathology such as a neurogenic bladder or posterior urethral valves are more likely to develop scars.
The hallmark of renal scarring is volume loss- either focal or global. These appear as wedge shaped defects or thinning and flattening of the cortex. Once scarring has occurred, no remedial or preventive measures can be taken and poor growth of scarred kidneys has been noted in children over 5 years of age. Renal scarring can result in hypertension- the increased risk of developing systemic HTN in later years in pediatric patients with renal scarring is approximately 10%--and if extensive, chronic renal failure. Jacobsen [2] in a 27 year follow-up of children with pyelonephritis found decreased renal function in all patients, hypertension in 33%, and end-stage renal disease in 10%. Prompt and effective treatment of acute pyelonephritis can prevent or markedly limit the extent of renal scarring, and therefore precise differentiation between upper and lower tract infection is vital for optimal management. [3]. Antibiotic prophylaxis can also be used to prevent recurrent infection.
Radiogaphic Imaging of Pyelonephritis
Tc-DMSA SPECT, spiral CT, and MR imaging are equally sensitive and reliable for the detection of acute pyelonephritis, power doppler imaging is significantly less accurate [10].
On plain film emphysematous pyelonephritis may be identified as air will be seen within the renal parenchyma, perirenal space, and the collecting system. IVP in patients with acute pyelonephritis is usually normal (75%), but a decreased or delayed nephrogram can be seen. The nephrogram may be patchy or striated and the involved kidney can be enlarged due to edema. Pelvocalyceal effacement may be seen due to compression by edematous parenchyma. The study generally reverts to normal following therapy
On Ultrasound the involved kidney most commonly has a normal appearance, although renal enlargement, loss of normally visualized pyramids, or decreased echogenicity (which may have a wedge-shaped appearance) have been described. Recently, power doppler has been used to evaluate for the presence of pyelonephritis with results similar to CT. Power doppler findings of pyelonephritis include triangular areas of decreased perfusion visible on both longitudinal and axial scans. The vasoconstriction associated with inflammation and compressive edema are probably responsible for the decreased perfusion identified on power doppler [4].
On helical CT during parenchymal phase images after IV contrast the affected kidney may appear swollen with a decreased nephrogram. Wedge-shaped areas of decreased attenuation and striation due to segmental vasospasm or pus-filled nephrons may be seen. On delayed enhanced scan, regions of pyelonephritis appear of increased attenuation in comparison to the normal kidney. Fascial thickening and blurring of the perinephric fat can also be seen in more severe cases.
MRI: Gadolinium-enhanced fast spin echo inversion recovery images have been used to evaluate for pyelonephritis. In this technique well perfused regions of the kidney have decreased signal intensity (ie: appear dark), while poorly perfused regions (ie: those involved by pyelonephritis) demonstrate increased (medium or high) signal intensity. This combination results in excellent lesion conspicuity. MR imaging can detect regions of pyelonephritis not identified at DMSA scintigraphy and is also better able to differentiate areas of scarring from areas of pyelonephritis [5].
Cortical Scintigraphy in Pyelonephritis and Renal Scarring:
Cortical scintigraphy is the procedure of choise for establishing the diagnosis of acute pyelonephritis [11].
Procedure:
The recommended minimum dose is 0.3 to 0.4 mCi of Tc-DMSA, with a maximum dose of 3 mCi (110 MBq). Imaging is performed 2 to 3 hours after injection and should include a posterior view, acquired for 300,000-500,000 counts or 5 minutes using a high-resolution parallel-hole collimator [11]. A posterior oblique view should also be acquired [11]. Pinhole images are then acquired using a 3-4 mm aperture for 100,000-150,000 counts or for 10 minutes [11]. SPECT images detect more defects, but are technically demanding, often require sedation, and offer no statistically significant advantage [6]. A recent report [7] showing SPECT defects in normal volunteers suggests specificity may decrease significantly with SPECT imaging. A study in piglets demonstrated slightly greater sensitivity, but lower specificity and equal accuracy for SPECT when compared with pinhole imaging [8]. For a detailed description, see SNM Nuclear Medicine Procedure Guidelines for Renal Cortical Scintigraphy in Children (June 1996). The estimated radiation dose to the kidneys (target organ) of a one year old child from 99m-Tc-DMSA is 0.78 mGy/MBq administered dose. A standard dose is about 1.85 MBq/kg (50uCi/kg). The radiation dose is therefore about 14.4 mGy (which is lower than that of spiral CT) [10].
Findings:
Differential renal functions are calculated from the parallel hole posterior image. When there is a significant depth discrepancy between the two kidneys, anterior and posterior images should be acquired and a geometric mean determined. The lowest normal value of relative uptake is 45% [11]. A greater than 10% difference in uptake between the two kidneys is considered abnormal [11]. Hydronephrosis may cause a problem with image interpretation because of tracer retention within the collecting system [11].
Because acute pyelonephritis can be focal, multifocal, or diffuse [11], there are 3 patterns seen with DMSA imaging:
- A single area of decreased cortical uptake with no evidence of volume loss
- Multiple unilateral or bilateral defects
- Diffuse involvement of an entire kidney
Cortical scintigraphy can detect twice as many cortical defects as ultrasound, and 4 times as many defects as IVP. Follow-up exams should be performed when healing of the acute lesion can be expected to be complete (about 6 months, and should probably not be performed within 3 months of an acute infection).
Scarring is the sequella of upper tract urinary infection and it is important to detect because of the potential risk for the subsequent development of systemic hypertension and renal impairment [11]. Acute pyelonephritis may cause residual scan abnormalities for at least 3 months after infection [11]. The rate of resolution of these defects is age dependent, clearing more slowly in infants and small children. A wait of between 3 to 6 months is reasonable prior to performing DMSA scanning to assess for scar, although improvement in scan abnormalities can be noted for up to 12 months after an episode of acute pyelonephritis [11]. Variability in exam interpretation is evident as interobserver agreement for the presence of renal scarring is about 77% [12].
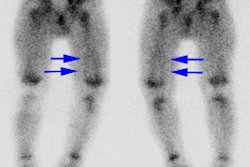
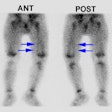
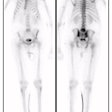
The case above demonstrates the cortical loss associated with renal scarring (left kidney)
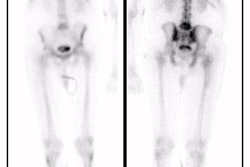
In this case the defects affect the upper and lower renal poles
Other agents used for cortical imaging/pyelonephritis imaging:
Glucoheptonate can also be used for renal cortical imaging and can produce diagnostic images, but unwanted collecting system activity presents problems for the Glucoheptonate exam. Furosemide, hydration, and delayed imaging may overcome this problem in patients with hydronephrosis. DMSA is also clearly superior to other agents such as gallium and In-111 WBC's for the evaluation of pyelonephritis in children. Gallium results in an inordinate delay to diagnosis and a larger radiation exposure. High splenic exposure and large blood volumes for labeling limit the use of radiolabeled WBC's in the pediatric population.
TcMAG3 images (when performed with lasix diuresis) will also demonstrate perfusion defects corresponding to areas of pyelonephritis- prolonged tracer retention (slow washout) can be seen in these areas [9].
REFERENCES:
(1) AJR 1996; 166: 1451-1455
(2) Br. Med. Journal 1989, 299; 703-6
(3) J.Urol 1983; Winberg et al. 129: 1190-94
(4) AJR 1996; 166: 1451-1455
(5) Radiology 1998; Lonergan GJ, et al. Childhood pyelonephritis: Comparison of gadolinium-enhanced MR imaging and renal cortical scntigraphy for diagnosis. 207: 377-384
(6) Semin Nucl Med 1993; Eggli DF, Tulchinski M. Scintigraphic evaluation of pediatric urinary tract infection. 23(3):199-218
(7) J Nucl Med 1996; DeSadeleer C, et al. Renal technetium-99m-DMSA SPECT in normal volunteers. 37(8):1346-9
(8) J Nucl Med 1996; Madj M, et al. Technetium-99m-DMSA renal cortical scintigraphy to detect experimental acute pyelonephritis in piglets: comparison of planar (pinhole) and SPECT imaging. 37(10):1731-4
(9) J Nucl Med 2000; Sfakianakis GN, et al. Diuretic MAG3 scintigraphy (Fo) in acute pyelonephritis: Regional parenchymal dysfunction and comparison with DMSA. 41: 1955-1963
(10) Radiology 2001; Majd M, et al. Acute pyelonephritis: Comparison of diagnosis with 99m-Tc-DMSA SPECT, spiral CT, MR imaging, and power doppler US in an experimental pig model. 218: 101-108
(11) J Nucl Med 2001; Rossleigh MA. Renal cortical scintigraphy and diuresis renography in infants and children. 42: 91-95
(12) J Nucl Med 2001; de Guevara DL, et al. Interobserver reproducibility in reporting on 99mTc-DMSA scintigraphy for detection of late renal sequelae. 42: 564-566