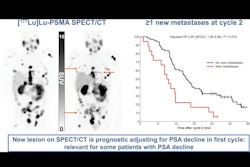
Lutetium-177 prostate-specific membrane antigen-617 (Lu-177 PSMA-617; Pluvicto, Novartis) appears to be on track as a first-line therapy for men with advanced prostate cancer.
After its initial approval in the U.S. in 2022 for cases that are resistant to any standard treatments, the drug recently met its primary endpoint in a new trial in men with metastatic, hormone-sensitive disease at the beginning of their diagnosis, explained Oliver Sartor, MD, of the East Jefferson General Hospital Cancer Center in New Orleans.
"This is an important trial because we're talking about the patient diagnosed yesterday [who] could be a candidate for this therapy tomorrow. You don't have to wait until you fail something like the other trials," he said.
The primary endpoint of the PSMAaddition trial was radiographic progression-free survival, which is used to assess the time during and after treatment when a patient's cancer does not worsen based on radiographic evidence, Sartor explained. Treatment also resulted in a positive trend in overall survival for patients, he added.
Importantly, Sartor noted, PSMA-PET scans play a key role in these settings, not only for identifying patients who have the disease, but in ongoing trials that could see the use of Lu-177 PSMA-617 for patients even earlier in the disease.
The nuts and bolts from the PSMAddition trial are expected to be presented at an upcoming medical meeting this Fall and subsequently submitted to the U.S. Food and Drug Administration in the second half of the year, Sartor noted.