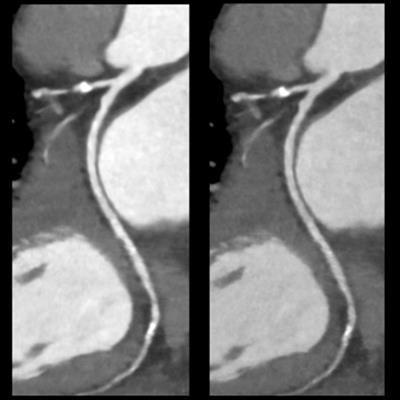
The U.S. Food and Drug Administration (FDA) has cleared Canon Medical Systems USA's deep-learning spectral CT software for cardiovascular applications for use with its Aquilion One/Prism Edition system.
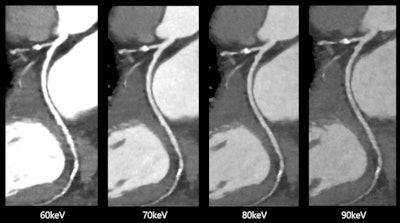 Spectral cardiovascular images produced by Canon Medical Systems' Aquilion One/Prism Edition CT with FDA-cleared deep learning software. Images courtesy of Canon Medical Systems.
Spectral cardiovascular images produced by Canon Medical Systems' Aquilion One/Prism Edition CT with FDA-cleared deep learning software. Images courtesy of Canon Medical Systems.The clearance makes it possible for Canon to market the scanner for use in acquiring 0.275-second whole-heart images in one heartbeat, with rapid kVp switching and deep-learning reconstruction of spectral CT images.




















