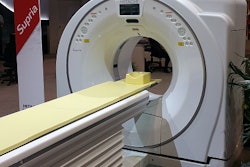
Hitachi Medical Systems America has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Supria 16-slice CT scanner.
Supria has a compact design and patient access features intended to accommodate a wide range of patient sizes. It is also compliant with the Smart Dose (XR-29) rules, according to the vendor. The scanner offers a 75-cm gantry aperture and a table with a 1.8-m scannable range and 500-lb weight capacity.
The system can be sited in a scan room as small as 200 sq ft, Hitachi said.