Endocarditis
Clinical:
Infective endocarditis is a rare, but potentially
life-threatening disease associated with substantial morbidity and
mortality [1]. The aortic valve is involved in 50% of cases [1,5].
It is most commonly seen in IV drugs users and in patients with
prothetic heart valves (approximately 5% of patients with
prosthetic heart valves develop endocarditis and involvement of
mitral valve prostheses is more frequent [4]) [2]. Causes of early
prosthetic valve endocarditis (PVE less than one year following
replacement) include perioperative contamination of the prosthesis
or cardiovascular instrumentation [9]. The most common organisms
are S aureus and S epidermidis which account for
40-50% of cases of early PVE [9].
Other predisposing conditions include calcific aortic stenosis,
congenital heart disease, HIV, and poor dentition [4]. Right sided
infective endocarditis accounts for 5-10% of all cases of and most
often invovles the tricuspid valve (isolated pulmonary valve
endocarditis is rare) [8]. Infectious vegetations form on the
valve cusps, most commonly on the ventricular surfaces of the
cusps [5]. Cutaneous manifestations result from peripheral
embolization and include petechiae, subungual splinter
hemorrhages, Osler nodes (painful transient subcutaneous nodules),
and Janeway lesions (nodular hemorrhages in distal digits [4].
Despite improvements in antibiotic therapy, surgery may be
undertaken in up to 50% of patients with IE [6]. The most frequent
indications for surgery are heart failure, perivalvular extension
with abscess formaiton, persistent sepsis, embolic events, and
large vegetation size, or a combination of factors [6].
Sterile endocarditis (nonbacterial thrombotic endocarditis) is
caused by immune complex deposition that precipitates an
inflammatory reaction and secondary thrombosis [2]. NBTE has been
reported in patients with advanced-stage malignancy
(adenocarcinoma of the colon, lung, ovary, orpancreas),
hematologic disrders, connective tissue disease, SLE (Libman-Sacks
endocarditis), AIDS, and hypercoaguable states [7,9]. The
vegetations in NBTE are dense, small (under 1 cm), broad based,
and irregular in shape [7]. Lambl excresences which occur as
filiform thrombi strands arising from the margins of the valve
leaflets are associated with nonbacterial endocarditis associated
with malignancy [9].
Complications:
Vegetation embolism- vegetations larger than 10mm in diameter have a 60% embolic incidence, compared to 23% for those smaller than 10 mm [1].
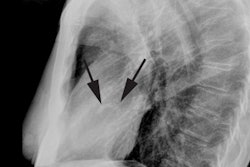
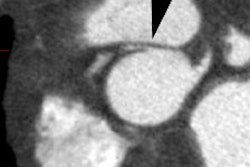
Perivalvular extension/perivalvular abscess/valvular aortic pseudoaneurysms- in 33-58% of patients, infective endocarditis of the aortic valve may extend into the annulus (perivaivular extension [6]) resulting in the formation of an aortic root abscess [1]. Perivalvular extension is common and affects 10-40% of patients with native valve IE and 56-100% of patients with prosthetic valve IE [6]. A perivalvular abscess may result in atrioventricular block or bundle branch block and persistent sepsis despite antibiotic use [1]. The presence of an aortic root abscess also complicates valve replacement and increases the risk for valve dehiscence [1]. An abscess does not communicate with the cardiac chambers [6]. A pseudoaneurysm does communicate with the intracardiac blood pool and will appear as a complex, pulsatile contrast filled perivalvular area on dynamic cine gated imaging [6]. Cardiac-gated CTA may be limited for the detection of small (4mm or less) vegetations and small valve perforations [6].
Valve perforations- small defects in the valve tissue that allow retrograde flow of blood into the preceding cardiac chamber [6].
X-ray:
Transesophageal echo: TEE is the most reliable means of
identifying vegetations because of their small size (2-3mm) [2].
The sensitivity and specificity of TEE in infective endocarditis
is 87-100% and 91-100%, respectively [3].
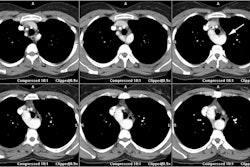
CT: Vegetations larger than 10 mm in diameter are almost always
detected by CT and are typically located on the ventricular side
of the aortic valve (low-pressure side of the valve) or the left
atrial side of the mitral valve [1,6]. The risk of embolization is
highest with large (>10mm) mobile vegetations [6].
PET: PET imaging has a limited role in the imaging of infective
endocarditis, however, valve uptake can be seen and resolve
following treatment [3].
REFERENCES:
(1) AJR 2010; Gahide G, et al. Preoperative evaluation in aortic
endocarditis: findings on cardiac CT. 194: 574-578
(2) AJR 2011; Hoey ETD, et al. Cardiovascular MRI for assessment
of infectious and inflammatory conditions of the heart. 197:
103-112
(3) J Nucl Cardiol 2011; Kenzaka T, et al. Positron emission
tomography scan can be a reassuring tool to treat difficult cases
of infective endocarditis. 18: 741-743
(4) Radiographics 2011; Kanne JP, et al. Beyond skin deep:
thoracic manifestations of systemic disorders affecting the skin.
31: 1651-1668
(5) Radiographics 2012; Bennett CJ, et al. CT and MR imaging of
the aortic valve: radiologic-pathologic correlation. 32: 1399-1420
(6) J Cardiovasc Comput Tomogr 2012; Entrikin DW, et al. Imaging
of infective endocarditis with cardiac CT angiography. 6: 399-405
(7) Radiology 2013; van Werkum MH, et al. Case 190: Papillary
fibroelastoma of the pulmonary valve. 266: 680-684
(8) J Cardiovasc Comput Tomogr 2015;
Passen E, PharmD ZF. Cardiopulmonary manifestations of isolated
pulmonary valve infective endocarditis demonstrated with cardiac
CT. 9: 399-405
(9) Radiographics 2016; Murillo H, et
al. Infectious diseases of the heart: pathophysiology, clinical
and imaging overview. 36: 963-983