Cardiac Transplant
- Clinical:
Survival from heart transplant in the modern era is over 90% at 1
year and the 5 year survival is over 80% [4]. The median survival
exceeds 10 years [4]. Newly transplanted hearts have chronotropic
incompetence due to cardiac denervation [7]. Transplant recipients
demonstrate higher resting heart rates due to loss of
parasympathetic innervation, yet decreased peak exercise heart
rates due to loss of sympathetic tone (and ultimately attenuated
exercise induced coronary flow reserve) [7].
Transplant related complications may be categorized into early
(0-30 days), intermediate (1-12 months), and late complications
(>12 months) [9].
Complications:
Infection:
Infection is the most common post-transplant complication and can
occur the early, intermediate, or late phase [9]. Bacteria account
for almost 44% of cases, and in more than half of the infections
are due to the gram negative bacteria E. coli and P. aeruginosa,
while Staph species are isolated in about a third of cases [9].
CMV infection affects nearly half of transplant recipients in the
first year (despite prophylaxis), but the majority of cases are
asymptomatic (active infection is seen in only 7.5% of patients)
[9]. In patients with active infection, CMV pneumonitis affects
9-11% of patients and has a mortality rate of 14% [9]. CT findings
include consolidation, patchy or diffuse ground glass opacities,
and centrilobular, tree-in-bud or random micronodules [9].
Graft failure:
Primary graft failure occurs in 2-25% of patients and is the most
common cause of death in the first 30 days following transplant
(responsible for up to about 40%) [9].
Cardiac allograft rejection:
Rejection represents the leading cause of mortality in the first year post transplant [6].
The risk for allograft rejection is greatest during the first
year following transplant, with a peak between weeks 2-12 [9].
Risk factors for rejection include young age, African American,
and female [9].
Hyperacute rejection typically occurs in the immediate post
transplant period and results from preformed recipient antibodies
directed against antigens expressed on the donor vascular
endothelium (typically human leukocyte antigens) [9]. The
condition is almost invariably fatal, but extremely rare due to
antigen screening [9].
Acute cellular rejection is the most common form of rejection-
20-40% of patients during the first year post transplant [9]. The
condition is a T cell-mediated process with leukocytes in the
myocardium. Acute antibody mediated rejection is less common-
occurring in 10-20% of patients in the first year [9]. Acute
antibody mediated rejection develops when recipient antibodies (de
novo donor specific antibodies may develop over time) are directed
against donor antigens, resulting in complement activation and
subsequent allograft dysfunction [9].
Endomyocardial biopsy (EMB) is the procedure of choice for
surveillance and diagnosis of rejection [9]. Histologic analysis
of the RV wall via endovascular biopsy is the gold standard for
diagnosis, but is invasive and carries a 0.5-1.5% complication
rate [6]. Delayed contrast enhanced MR is a non-invasive imaging
modality that can be used to assess for acute rejection [6]. Other
authors suggest that increased T2 signal in the myocardium
correlates with moderate acute rejection with a sensitivity of 89%
and a specificity of 70% [9].
Coronary artery/allograft vasculopathy (CAV):
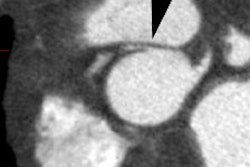
CAV is an inflammatory vasculopathy distinct from traditional atherosclerotic coronary artery disease [7] and represents a late complication of transplant (> 1 year) [9]. CAV is characterized by the presence of an intact elastic lamina and a diffuse concentric disease affecting the entire coronary tree with intimal hyperplasia composed of vascular smooth muscle cells (it is defined as an accelerated fibrous intimal hyperplasia within donor coronary arteries that manifests with smooth muscle proliferation, lipid deposition, and inflammatory cell accumulation in the walls of the coronary arteries) [8,9]. CAV develops in 8-10% of cardiac transplant patients at one year post transplant, 30-50% by 5 years, and in over 50% of patients at 10 years [1,4]. Anginal symptoms are usually lacking due to cardiac denervation and the first clinical signs are frequently severe with heart failure, infarction, ventricular arrhythmia, or sudden death [2,4,7]. CAV progresses rapidly [4]. The diffuse nature of the process renders conventional coronary revascularization less useful and re-transplantation is the only definitive therapy [1].
CAV is a leading cause of long term graft failure, need for
re-transplantation, and death [7]. The mortality is between
10-20% within 1 year of diagnosis [4,7] and CAV is the most common
cause for late-stage mortality in heart transplant recipients [5].
Risk factors for CAV include the number of HLA mismatches with the
allograft and the number of rejection episodes [4].
Alloantigenic-indpendent risk factors include traditional cardiac
risk factors such as HTN, dyslipidemia, DM, and obesity) and CMV
infection [4,8]. Statin therapy decreases CAV and improves
survival [4]. Proliferation signal inhibitors (sirolimus,
everolimus) also decrease the progression of CAV [4].
Interestingly, regular exercise has also been shown to reduce the
progression of CAV [7].
Currently, CAV is felt to be related to an inflammatory process
in the spectrum of chronic rejection and the result of
immunological differences between the graft and host, leading to
immunologically mediated arterial hyperplasia [1,6]. Early
diagnosis is important because this may allow modified
immunosuppression therapy and judicious percutaneous intervention
of areas of focal stenosis [6]. The disease is most commonly
characterized by concentric and diffuse intimal thickening of the
middle and distal segments of the epicardial vessels (as well as
distal branch coronary arteries) and thus may be underestimated by
coronary angiography [1,4,5,7]. CT angiography allows for
visualization of the coronary vessel walls [9]. Studies have shown
that 30-50% of coronary segments classified as normal with
conventional angiography show wall thickening at CT angiography
[9]. Intravascular US is the most sensitive imaging modality for
the diagnosis of CAV [9]. The condition is diagnosed when intimal
thickness is greater than 0.3mm [9]. An intimal thickness greater
than 0.3mm at 1 year after transplant is associated with a 23%
decreased survival rate (96% vs 73%) [9].
Valvular complications:
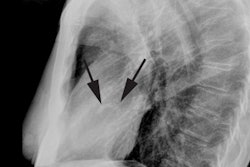
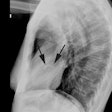
Tricuspid regurge is the most common valvular complication after
OHT, with a prevalence of 19-84% [9]. Many surgical centers
perform tricuspid annuloplasty at the time of transplant, which
decreases the incidence of regurge [9].
Malignancy:
Malignancy is a leading cause of morbidity and mortality in the
late post transplant period- developing in 16% of survivors at 5
years, and 28% of survivors at 10 years [9].
Skin cancer is the most common malignancy affecting OHT patients-
and it develops in nearly 10% of patients within the first 5
years, most commonly basal cell and squamous cell [9]. After skin
cancer, lung cancer is the most common malignancy in OHT patients,
most commonly non-small cell [9]. The incidence of post transplant
lung cancer is 1.86 times higher than the general population [9].
Risk factors include male sex and prior smoking [9].
PTLD and/or lymphoma is the second most common malignancy in OHT
patients [9]. Pediatric and young adult patients have a markedly
increased risk for PTLD and lymphoma (patients age 18-35 years
have a 27 x increased risk compared to non-transplant control
patients), possibly due to a lack of protection against oncogenic
viruses such as Ebstein-Barr [9]. An EBV positive donor in an EBV
negative pediatric recipient constitutes a strong risk factor for
PTLD [9].
-X-ray:
Coronary angiography has served as the primary imaging modality
for CAV diagnosis [7]. The major limitation of angiography is its
limited sensitivity in identifying eraly, small-vessel, or even
advanced diffuse CAV [7]. The diffuse luminal narrowing of
advanced CAV leads to difficulties in identifying reference
segments and branch vessel obliteration may go unrecognized [7].
Intravascular US is the most sensitive imaging modality for the
detection of CAV (CAV is defined as [maximial intimal thickness] -
[intima and media thickess] of greater than 0.5 mm) [4]. The
presence of severe intimal thickening ≥ 0.5 mm in the first
year after transplant by IVUS predicts cardiac events, graft loss,
and death [7]. Coronary angiography is known to underestimate CAV
when compared to IVUS [6].
The presence of coronary calcium indicates the presence of CAV
and is a strong predictor of future cardiac events, however, CAC
is an insensitive marker for CAV and can be absent in 6% of
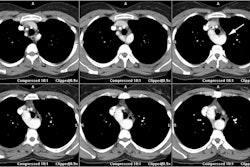
patients with substantial CAV at angiography [5,6]. Multidetector
coronary CT has an advantage over conventional angiography for the
detection of CAV (the diffuse nature of the process may hinder
it's identification by angiography) [1,4]. Difficulties with CTA
imaging may occur with elevated HR's and lack of response to
beta-blockers in post-cardiac transplant patients [4].
Retrospective gating can be used, but this will increase patient
radiation exposure [5]. The reported sensitivity of CTA for CAV on
a per segment (and per patient) basis is 83% (86%), specificity
99% (89%), PPV 76% (67%) and NPV 99% (96%) [4].
SPECT myocardial perfusion imaging can detect perfusion
abnormalities secondary to CAV [2]. The reported sensitivity is
84%, specificity 70%, and NPV at 12 months is 95-98% [6]. Because
of the diffuse nature of CAV, SPECT MPI may underestimate the
severity due to image normalization errors [6]. Dobutamine stress
can utilized instead of exercise because of the blunted heart rate
response to exercise common in post cardiac transplant patients
[6]. Adenosine is not commonly used in heart transplant patients
given concerns for AV block and sinus pauses or arrest that can
occur due to adenosine supersensitivity in denervated hearts
(although the effects are typically transient) [7,8]. Compared to
adenosine, regadenoson has been shown to cause less conduction
abnormalities in patients that are several years post-transplant
(its safety in the early post-transplant period has not been fully
evaluated) [6].
However, using dynamic PET to accurately determine myocardial
blood flow, impaired coronary flow reserve can be detected in up
to 20% of patients with normal semiquantitative imaging [3]. Note
that resting MBF is higher in heart transplant patients compared
to controls likely due to increasing resting heart rate and
rate-pressure product in the denervated heart [8].
For the diagnosis of CAV (>50% stenosis), coronary CTA has a
mean weighted sensitivity of 94%, specificity of 92%, NPV of 99%,
PPV of 67%, and a diagnostic accuracy of 94% [7]. CCTA is limited
for the evaluation of small coronary vessels and motion artifacts
can degrade the exam, particularly in association with the high
heart rates that are commonly encountered with denervated hearts
[7].
REFERENCES:
(1) J Nucl Cardiol 2010; Soman P, et al. Surveillance for post-transplant coronary artery vasculopathy: shifting gears from diagnosis to prognosis. 17: 172-174
(2) J Nucl Cardiol 2010; Manrique A, et al. Diagnostic and prognostic value of myocardial perfusion gated SPECT in orthotopic heart transplant recipients. 17: 197-206
(3) J Nucl med 2010; Wu YW, et al. PET assessment of myocardial
perfusion reserve inversely correlates with intravascular
ultrasound findings in angiographically normal cardiac transplant
recipients. 51: 906-912
(4) J Cardiovasc Comput Tomogr 2012; Ferencik M, et al. Computed
tomography imaging of cardiac vasculopathy. 6: 223-231
(5) Radiology 2013; Mittal TK, et al. Cardiac allograft
vasculopathy after heart transplantation: electrocardiographically
gated cardiac CT angiography for assessement. 268: 374-381
(6) J Nucl Cardiol 2015; Gupta B, et al. Imaging in patients
after cardiac transplantation and in patients with ventricular
assist devices. 22: 617-638
(7) J Nucl Cardiol 2016; Payne GA, et al. Transplant allograft
vasculopathy: role of multimodality imaging in surveillance and
diagnosis. 23: 713-727
(8) J Nucl Cardiol 2017; Sevag RR, Maddahi J. Regadenoson-induced
hyperemia for absolute myocardial blood flow quantitation by
13N-ammonia PET and detections of allograft vasculopathy. 24:
1145-1148
(9) Radiographics 2019; Smith JD, et al. Evaluation after orthotopic heart transplant: what the radiologist should know. 39: 321-343