Bone Imaging of Vascular Disorders
Avascular Necrosis:
There are numerous etiologies for avascular necrosis, including trauma (most common)
and non-traumatic causes such as steroids, sickle cell disease, caisson disease,
vasculitis (XRT,SLE), ETOH, pancreatitis, and Gaucher's disease. When no etiology is
determined the disorder is idiopathic (about 25% of cases). Commonly involved bones
include the femoral and humeral heads, and the talus.
Legg-Calve-Perthes
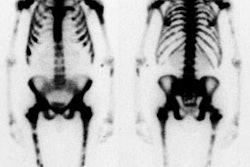
Idiopathic ischemic necrosis of the femoral head in children is also referred to as Legg-Calve-Perthes disease. This disorder is most commonly seen in children between the ages of 4 to 9 years. It is more common in males (5:1) and is uncommon in blacks. Bilateral hip involvement is uncommon (10%) and usually sequential (asymmetric). Patients typically present with pain in the affected hip and a limp. No definitive risk factors have been described although associations with congenital heart disease, pyloric stenosis, renal anomalies, undescended testis, and a family history have been proposed. Prognosis is in part dependent upon the age of the patient (a younger age at onset is associated with a better prognosis) and extent of involvement of the femoral head (greater than 50% involvement is associated with a worse prognosis). Complications include premature osteoarthritis, osteochondritis dissecans, and infection in Sickle cell patients.
On plain film, the earliest finding of Legg-Calve-Perthes is evidence of a joint effusion (widened teardrop distance). A decreased size of the femoral capital epiphysis is seen in about half of the cases, but it is not evident until several weeks after the insult. A "crescent sign" is the most reliable radiographic finding, representing a subchondral fracture. Other findings include a metaphyseal lucency or cyst (felt to be the result of intramedullary hemorrhage due to stress fractures) and a broad short femoral neck. Later there is increased density within the capital epiphysis due to new bone formation. With non-healing, there will be collapse of the femoral head with flattening, sclerosis, and distortion of the articular surface.
On plain films, there is a five stage grading system (FICAT Staging) for femoral head AVN:
- Normal radiograph (MRI or bone scan will be abnormal)
- Increased density/sclerosis
- Crescentic lucency
- Femoral head irregularity
- Collapse
- Degenerative change
On MRI, there is loss of the normal high T1 signal within the capital epiphysis/femoral head, as well as an increased signal on T2-weighted imaging. These patterns may be band-like or patchy on both T1 and T2-weighted images.
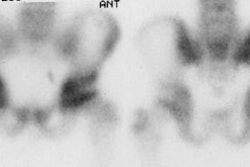
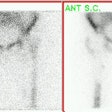
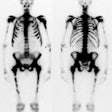
For scintigraphic evaluation, studies are best performed early after the onset of clinical symptoms, since later scans often demonstrate increased activity due to revascularization and remodeling or secondary degenerative changes. Early bone scanning has a reported sensitivity and specificity of greater than 90% in the diagnosis of AVN. SPECT imaging is superior to planar images in identifying a photon deficient area in the femoral head. Unfortunately in children, the small size of the epiphysis and the surrounding activity in the acetabulum limit the usefulness of SPECT. Thus, in children, pinhole images should be performed to improve resolution. To obtain optimal images, a 2 mm aperture collimator should be brought to a distance in which the femoral head and neck occupy the central portion of the image and the bladder should be excluded from the field of view. A minimum of 100,000 (to 150,000) counts is recommended for pinhole images. In general, once the radiographic signs have established the presence of AVN, the bone scan is unlikely to add additional information.
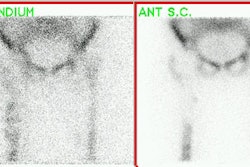
Sulfur colloid imaging can also be used for the evaluation of avascular necrosis in young individuals due to the abundance of reticuloendothelial cells within the femoral heads.
4 stages of AVN on bone scan [Gottschalk]:
Stage 1
The first, or early stage, demonstrates absent activity in all or a portion of the femoral head on delayed images due to interruption of the vascular supply. Radiographs are generally normal at this time. This finding is usually observed in patients who have been symptomatic for less than 1 month. Decreased activity in the femoral capital epiphysis has also been reported with septic arthritis, transient synovitis, and post-traumatic hemarthrosis as fluid within the joint may raise the pressure within the joint to such an extent that it impairs blood flow producing the cold defect. The finding is generally reversed in these disorders following arthrocentesis which relieves the intracapsular pressure.
Stage 2
The second stage reflects early revascularization and usually occurs within 1 to 4 months. Repair is initiated first by hyperemia and the formation of reactive granulation tissue at the interface with normal bone. Revascularization of the dead tissue begins a few weeks later by in-growth of vessels from the surrounding reactive tissue. Progressive infiltration of the necrotic area from the periphery by collateral vessels results in laying down of new trabeculae and the resorption of old necrotic ones. It is during this stage that the radiographs demonstrate increased density within the femoral head. Focal intense Tc-MDP accumulation usually corresponds to this area, but may be seen even before the repair process has produced sufficient sclerosis to be visible on the radiograph. Another characteristic finding is that of a focus of decreased tracer accumulation (representing central necrosis) surrounded by a rim or crescent of increased activity. Occasionally, patients may have a normal scan during this stage as the lesion transitions from a photon-deficient abnormality to one with focally increased activity.
Stage 3
As healing progresses, the focal area of increased tracer activity progressively extends into the necrotic zone and eventually results in diffuse increased activity within the femoral head, beginning about 10 months following the insult.
Stage 4
The final stage represents a return to normal activity with uncomplicated healing. If healing does not occur, then increased activity on both sides of the joint reflects the presence of degenerative changes.
Bone Infarct:
There are 2 major forms of bone infarction: 1- Medullary bone infarction which involves the trabecular architecture and marrow cavity and is usually clinically silent; and 2- Corticomedullary infarction which is typically subchondral, painful, and classically involves the femoral or humeral heads. Pathologically, medullary infarcts are non-progressive. When they involve fatty marrow, insoluble soaps are formed by the interaction of released calcium and fat. These abnormalities persist for life. Bone marrow scanning is more sensitive than bone scan for the detection of bone infarction/aseptic necrosis. Unfortunately, the sulfur colloid prepared with commercially available kits has rather large sized particles (0.1-0.6 microns) which results in predominant localization to the liver and spleen, and only limited marrow activity. Additionally, the distribution of marrow in the appendicular skeleton in individuals over the age of 15 is limited to the proximal portions of the femurs and humeri, and thus marrow scintigraphy is of little value in the detection of more distal infarctions. In patients with hemoglobinopathies, however, there is distal expansion of the red marrow which makes evaluation of more distal infarctions possible.
Acutely (1st week) following bone infarction there will be a marrow defect on Tc-SC imaging that corresponds to an area of decreased tracer activity on the bone scan. The marrow study typically demonstrates a more extensive area of infarction than evident on bone scintigraphy. Later (beyond 1 week), increased tracer accumulation may be seen on the bone scan at the site of infarction due to revascularization and the presence of reactive bone about the area. This abnormality may remain positive for several months. A persistent defect on the marrow scan can be seen if the area of infarction became fibrosed, rather than repopulated.
On gallium scanning, acutely there is decreased activity at the site of infarction. Chronic infarctions can have variable uptake of the tracer, but the intensity of the abnormality should be less than or equal to that noted on the bone scan. The contralateral normal side is used as a reference to determine the intensity of the abnormality. The uptake should also be congruent in size to the bone scan abnormality.
Radiation Osteonecrosis:
Radiation damage to bone is primarily mediated by alterations in the microvasculature. With doses above 3000 rads delivered over 3 weeks, there is a detectable depression in marrow and bone uptake of Tc-SC and Tc-MDP, respectively. For any given radiation exposure, the depression of colloid uptake is generally more marked than the depression of bone tracer uptake. In fact, depressed marrow uptake (both of Tc-SC and In-111 WBC's) can be seen with doses as small as a few hundred rads. As the absorbed dose increases, so does the incidence of irreversible osteonecrosis.
Within hours following bone irradiation, increased uptake of Tc-MDP can be seen most likely related to a reactive hyperemia and increased vascular permeability. The reduction in activity on bone scan following XRT is generally not evident for 3 to 6 months, but may persist for many years, or possibly for life. Irradiated bone is also more susceptible to fracture and infection.