Bone Imaging of
Vascular Disorders
Avascular Necrosis (Osteonecrosis):
Osteonecrosis typically occurs in patients 20-50 years of age [10]. The hps and knees are reported to be the joints most commonly involved [10]. There are numerous etiologies for avascular necrosis, including trauma (most common) and non-traumatic causes such as steroids, sickle cell disease, caisson disease, vasculitis (XRT,SLE), ETOH, pancreatitis, bisphosphonate use, and Gaucher's disease. Osteonecrosis can also affect between 5% to 29% of renal transplant recipients and the femoral head is the most common site [2]. Childhood cancer survivors are also at an increased risk for developing osteonecrosis- particularly those with a history of acute lymphocytic leukemia and those who have undergone allogeneic bone marrow transplantation [10]. When no etiology is determined the disorder is idiopathic (about 25% of cases). Commonly involved bones include the femoral and humeral heads, and the talus.
In adults, early detection of femoral head AVN is essential as surgical core decompression may arrest the progress of the condition and prevent subsequent femoral head collapse [2]. Unfortunately, osteonecrosis often remains asymptomatic or associated with only minimal non-specific symptoms until is becomes advanced [10].
MR imaging is the most sensitive and specific method for detection and monitoring of osteonecorsis [10]. Scinitgraphy has been shown to be superior to MR for the detection of early femoral head AVN in certain clinical situations [2]. Spontaneous osteonecrosis can be seen in elderly patients and is characterized by the abrupt onset of knee pain with normal radiographs [3]. On bone scan, spontaneous osteonecrosis is characterized by focally increased tracer uptake, most commonly in the medial femoral condyle [3].
Bisphosphonate osteonecrosis (Osteonecrosis
of the jaw)
Bisphosphonates are non-metabolized synthetic compounds with a structure similar to inorganic pyrophosphate [4]. The compounds have a great capacity for binding to the bone matrix where they act to inhibit osteoclastic action and decrease bone turnover [4,5,9]. As the agents are not metabolized, high concentrations remain in the bone for extended periods of time [9]. The agents are used in the treatment of Paget's disease, lytic bone metastases, multiple myeloma, and osteoporosis to reduce bone pain, demineralization, and fractures [4].
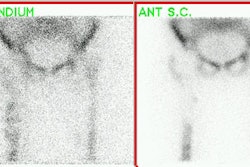
Osteonecrosis of the jaw (ONJ) is a complication of bisphosphonate treatment in agents with nitrogen in their structure (pamidronate, zoledronic acid, and alendronate) [4]. The prevalence of ONJ has been reported to be between 1-10% in patients receiving IV bisphosphonate treatment for cancer [9]. The prevalence in patients treated for osteoporosis (which is typically treated with a less powerful bisphosphonate) is between 0.0004 and greater than 0.04 % [9]. The condition most commonly involves the mandible (2/3's of cases), but it can also involve the maxilla (25% of cases), or both mandible and maxilla [5,9]. ONJ can manifest 6-60 months after intiation of bisphosphonate treatment [9]. ONJ is generally preceded by a tooth extraction, although no preceding event is identified in up to 20% of cases [4]. Potential reported associated risk factors include poor oral hygiene, alcohol or tobacco use, increased age, diabetes, and immunosuppression [9]. Most patients present with jaw pain and exposed necrotic bone [5]. ONJ usually involves the mandible, but the maxilla can also be involved [7]. The lesion generally involves both the bone cortex and bone marrow, and adjacent soft tissue changes are also commonly seen on MR (57% of cases) [4]. ONJ can also be seen following radiation therapy for head and neck malignancies [5].
Treatment for ONJ includes oral antimicrobial rinse and systemic antibiotic therapy [9]. Areas of necrotic bone that are a source of soft tissue irritation should be recontoured and loose segments of bone sequestrum should be removed [9]. Prevention is probably the best treatment, and dental extraction or dental implants should be avoided in patients taking bisphosphonate therapy [9].
On plain film and CT the appearance of ONJ is variable and includes ill-defined areas of lucency, permeative appearance, cortical destruction, bony sequestrum (more advanced stage), periosteal reaction (more advanced stage), sclerotic changes, or a mixed pattern [9]. A persistent nonhealing alveolar socket after a tooth extraction has also been described as a typical radiographic feature [9].
On bone scan, osteonecrosis of the jaw demonstrates increased flow, blood pool and delayed tracer activity corresponding to the area of osteonecrosis [7]. Increased uptake is noted in 60% to more than 90% of cases [9]. SPECT imaging aids in detection and localization of areas of osteonecorsis [7].
On MRI ONJ is typically associated with decreased signal on T1, while there
is greater variability in the appearance on T2 or STIR images [9].
Legg-Calve-Perthes
Idiopathic ischemic necrosis of the femoral head in children is also referred to as Legg-Calve-Perthes disease. This uncommon disorder is most commonly seen in children between the ages of 4 to 9 years (peak 5-6 years of age [8]. It is more common in males (5:1) and is uncommon in blacks. Bilateral hip involvement is uncommon (10-15%) and usually sequential (asynchronously [8]). Patients typically present with pain in the affected hip and a limp. No definitive risk factors have been described although associations with congenital heart disease, pyloric stenosis, renal anomalies, undescended testis, and a family history have been proposed. Prognosis is in part dependent upon the age of the patient (a younger age at onset is associated with a better prognosis) and extent of involvement of the femoral head (greater than 50% involvement is associated with a worse prognosis). Complications include premature osteoarthritis, osteochondritis dissecans, and infection in Sickle cell patients. The disorder can be divided into 3 phases- the avascular phase, the revascularization phase, and the reparative phase [8]. The avascular phase typically lasts several months and is commonly painful and associated with a limp [8]. Following the avascular phase, necrotic bone is eventually resorbed and replaced by granulation tissue [8]. With time, granulation tissue is replaced by more mature fibrous tissue, cartilage, and eventually, mature trabecular bone [8].
On plain film, the earliest finding of Legg-Calve-Perthes is evidence of a joint effusion (widened teardrop distance). A decreased size of the femoral capital epiphysis is seen in about half of the cases, but it is not evident until several weeks after the insult. A "crescent sign" is the most reliable radiographic finding, representing a subchondral fracture. Other findings include a metaphyseal lucency or cyst (felt to be the result of intramedullary hemorrhage due to stress fractures) and a broad short femoral neck. Later there is increased density within the capital epiphysis due to new bone formation. With non-healing, there will be collapse of the femoral head with flattening, sclerosis, and distortion of the articular surface.
On plain films, there is a five stage grading system (FICAT Staging) for femoral head AVN:
- Normal
radiograph- early in the avascular phase the
plain film radiograph is normal, but MRI or bone scan will be abnormal
- Increased
density/sclerosis
- Crescentic
lucency
- Femoral
head irregularity
- Collapse
- Degenerative
change
On MRI, there is loss of the normal high T1 signal within the capital epiphysis/femoral head, as well as an increased signal on T2-weighted imaging. These patterns may be band-like or patchy on both T1 and T2-weighted images.
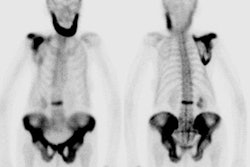
For scintigraphic evaluation, studies are best performed early after the onset of clinical symptoms, since later scans often demonstrate increased activity due to revascularization and remodeling or secondary degenerative changes. Early bone scanning has a reported sensitivity and specificity of greater than 90% in the diagnosis of AVN. SPECT imaging is superior to planar images in identifying a photon deficient area in the femoral head. Unfortunately in children, the small size of the epiphysis and the surrounding activity in the acetabulum limit the usefulness of SPECT [1]. Bladder activity can also create artifacts on reconstruction images [1]. Thus, in children, pinhole images should be performed to improve resolution. To obtain optimal images, a 2 mm aperture collimator should be brought to a distance in which the femoral head and neck occupy the central portion of the image and the bladder should be excluded from the field of view. A minimum of 100,000 (to 150,000) counts is recommended for pinhole images. An image of the opposite hip should also be obtained using the same time interval. In general, once the radiographic signs have established the presence of AVN, the bone scan is unlikely to add additional information.
Sulfur colloid imaging can also be used for the evaluation of avascular necrosis in young individuals due to the abundance of reticuloendothelial cells within the femoral heads.
4 stages of AVN on bone scan
[Gottschalk]:
Stage 1
The first, or acute stage, demonstrates absent activity in all or a portion of the femoral head on delayed images due to interruption of the vascular supply. Radiographs are generally normal at this time. This finding is usually observed in patients who have been symptomatic for less than 1 month. Decreased activity in the femoral capital epiphysis has also been reported with septic arthritis, transient synovitis, and post-traumatic hemarthrosis as fluid within the joint may raise the pressure within the joint to such an extent that it impairs blood flow producing the cold defect. The finding is generally reversed in these disorders following arthrocentesis which relieves the intracapsular pressure.
Stage 2
The second stage reflects early revascularization and usually occurs within 1 to 4 months. Repair is initiated first by hyperemia and the formation of reactive granulation tissue at the interface with normal bone. Revascularization of the dead tissue begins a few weeks later by in-growth of vessels from the surrounding reactive tissue. Progressive infiltration of the necrotic area from the periphery by collateral vessels results in laying down of new trabeculae and the resorption of old necrotic ones. It is during this stage that the radiographs demonstrate increased density within the femoral head. Focal intense Tc-MDP accumulation usually corresponds to this area, but may be seen even before the repair process has produced sufficient sclerosis to be visible on the radiograph. Another characteristic finding is that of a focus of decreased tracer accumulation (representing central necrosis) surrounded by a rim or crescent of increased activity. Occasionally, patients may have a normal scan during this stage as the lesion transitions from a photon-deficient abnormality to one with focally increased activity.
Stage 3
As healing progresses, the focal area of increased tracer activity progressively extends into the necrotic zone and eventually results in diffuse increased activity within the femoral head, beginning about 10 months following the insult.
Stage 4
The final stage represents a return to normal activity with uncomplicated healing. If healing does not occur, then increased activity on both sides of the joint reflects the presence of degenerative changes.
|
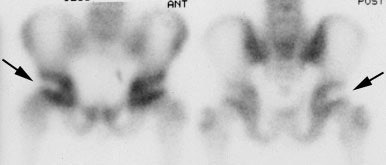
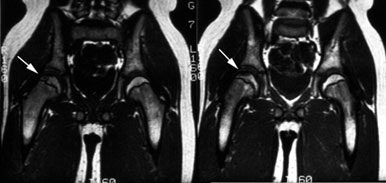
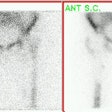
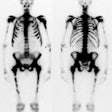
Legg-Calve-Perthes: The child shown below was being evaluated for right hip pain. The bone scan demonstrated a focal area of photopenia in the lateral aspect of the right femoral head (black arrow) consistent with avascular necrosis. (Click the planar images to view selected SPECT images). T1-weighted MR images from the same patient demonstrate signal loss in the right femoral head epiphysis. A focal area of signal void can be seen in the lateral aspect of the epiphysis. (Click MR image to enlarge) |
|
|
Bone
Infarct:
There are 2 major forms of bone infarction: 1- Medullary bone infarction which involves the trabecular architecture and marrow cavity and is usually clinically silent; and 2- Corticomedullary infarction which is typically subchondral, painful, and classically involves the femoral or humeral heads. Pathologically, medullary infarcts are non-progressive. When they involve fatty marrow, insoluble soaps are formed by the interaction of released calcium and fat. These abnormalities persist for life. Bone marrow scanning is more sensitive than bone scan for the detection of bone infarction/aseptic necrosis. Unfortunately, the sulfur colloid prepared with commercially available kits has rather large sized particles (0.1-0.6 microns) which results in predominant localization to the liver and spleen, and only limited marrow activity. Additionally, the distribution of marrow in the appendicular skeleton in individuals over the age of 15 is limited to the proximal portions of the femurs and humeri, and thus marrow scintigraphy is of little value in the detection of more distal infarctions. In patients with hemoglobinopathies, however, there is distal expansion of the red marrow which makes evaluation of more distal infarctions possible.
Acutely (1st week) following bone infarction there will be a marrow defect on Tc-SC imaging that corresponds to an area of decreased tracer activity on the bone scan. The marrow study typically demonstrates a more extensive area of infarction than evident on bone scintigraphy. Later (beyond 1 week), increased tracer accumulation may be seen on the bone scan at the site of infarction due to revascularization and the presence of reactive bone about the area. This abnormality may remain positive for several months. A persistent defect on the marrow scan can be seen if the area of infarction became fibrosed, rather than repopulated.
On gallium scanning, acutely there is decreased activity at the site of infarction. Chronic infarctions can have variable uptake of the tracer, but the intensity of the abnormality should be less than or equal to that noted on the bone scan. The contralateral normal side is used as a reference to determine the intensity of the abnormality. The uptake should also be congruent in size to the bone scan abnormality.
Radiation Osteonecrosis:
Radiation damage to bone is primarily mediated by alterations in the microvasculature with ischemic changes that weaken the bone [6]. Irradiation also kills osteoblasts, osteocytes, and osteoclasts- leaving an acellular bone matrix [6]. The reduction in osteoblastic cells after irradiation results in decreased collagen production and alkaline phosphatase activity [6]. The effects of radiation osteitis are usually only seen when doses of at least 1750 to 3000 rads are delivered (ie: a detectable depression in marrow and bone uptake of Tc-SC and Tc-MDP, respectively) [3]. For any given radiation exposure, the depression of colloid uptake is generally more marked than the depression of bone tracer uptake. In fact, depressed marrow uptake (both of Tc-SC and In-111 WBC's) can be seen with doses as small as a few hundred rads. As the absorbed dose increases, so does the incidence of irreversible osteonecrosis.
Early and late phase findings can be observed following radiation therapy [3]. Within hours following bone irradiation, increased uptake of Tc-MDP can be seen most likely related to a reactive hyperemia and increased vascular permeability [3]. This increased activity, which peaks about 2-3 months after treatment, is only about 10-20% above baseline and may not be appreciated at visual image inspection [3].
Late phase changes are characterized by uniformly decreased tracer uptake within the radiation port [3]. The reduction in activity on bone scan following XRT is generally not evident for 3 to 6 months, but may persist for many years, or possibly for life. Irradiated bone is also more susceptible to fracture and infection.
REFERENCES:
(1) Semin Nucl Med 1993; Conway JJ. A scintigraphic classification of Legg-Calve-Perthes disease. 23: 274-295
(2) J Nucl Med 2002; Ryu JS, et al. Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. 43: 1006-1011
(3) Radiographics 2003; Love C, et al. Radionuclide bone imaging: an illustrative review. 23: 341-358
(4) AJR 2008; Garcia-Ferrer L, et al. MRI of mandibular osteonecrosis secondary to bisphosphonates. 190: 949-955
(5) Am J Neuroradiol 2006; Carneiro E, et al. Bisphosphonate-associated mandibular osteonecrosis. 27:1096-1097
(6) AJR 2008; Kwon JW, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. 191: 987-994
(7) J Nucl Med 2009; Dore F, et al. Bone scintigraphy and SPECT/CT of biphosphonate-induced osteonecrosis of the jaw. 50: 3-35
(8) AJR 2009; Dillman JR, Hernandez RJ. MRI of Legg-Calve-Perthes disease. 193: 1394-1407
(9) Radiographics 2009; Morag Y, et al. Bisphosphonate-related osteonecrosis of the jaw: a pictorial review. 29: 1971-1986
(10) AJR 2011; Kaste SC, et al. Osteonecrosis in children after therapy for malignancy. 196: 1011-1018