Sonex Health and the Institute of Advanced Ultrasound Guided Procedures recently enrolled the first patient in a multicenter prospective trial for the company's office-based, ultrasound-guided carpal tunnel release technique.
The "Robust" trial aims to report the safety and effectiveness of Sonex Health's UltraGuideCTR technology in patients with symptomatic carpal tunnel syndrome. Dr. Victor Marwin with Bluegrass Orthopaedics in Lexington, KY, was the first investigator to consent a patient into the study, and Dr. James Watt with Orthopaedic Associates in Fort Walton Beach, FL, was the first to enroll and treat a patient in the study.
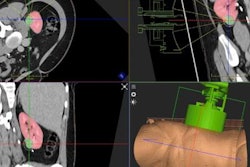
UltraGuideCTR enables physicians to use a minimally invasive technique while performing carpal tunnel release through a small wrist incision, according to Sonex. This method is performed under local anesthesia and is appropriate for an office-based setting rather than hospitals, the firm said.
The study aims to enroll 140 patients, including treating patients with bilateral carpal tunnel syndrome and following those subjects for two years post treatment. It is being led by Dr. Ashley Pistorio from the University of Nevada in Las Vegas.