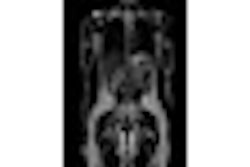
Breast MRI technology developer Aurora Imaging Technology has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its AuroraSpectroscopy breast MR spectroscopy package.
The availability of AuroraSpectroscopy enables Aurora's 1.5-tesla dedicated breast MRI system to perform in vivo breast MR spectroscopy and MR spectroscopic imaging, according to the North Andover, MA-based firm.
Related Reading
Road to RSNA, MRI, Aurora Imaging Technology, October 31, 2008
Road to RSNA, Women's Imaging, Aurora Imaging Technology, October 28, 2008
Aurora completes Loma Linda installation, October 24, 2008
Aurora adds Georgia install, September 22, 2008
Aurora adds PA customer, September 15, 2008
Copyright © 2008 AuntMinnie.com