Bone Imaging in the Post-operative Patient:
Post-operative Evaluation of Spinal Fusion:
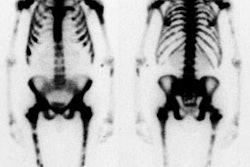
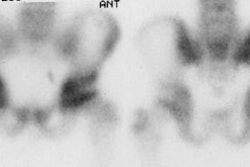
Spinal fusion is performed in patients with back pain due to segmental instability. Persistent back pain in the first few years after the procedure is often related to failure to achieve structural integrity of the fusion (pseudoarthrosis). The incidence of pseudoarthrosis following spinal fusion varies from 10 to 30%, however, not all patients with pseudoarthrosis suffer from back pain. Early failed fusion is usually treated with repeat surgery. Bone scanning can be used to detect the presence of a pseudoarthrosis in patients with continued pain following spinal fusion. On SPECT images successful fusions demonstrate diffuse, but not increased, uptake which can be inhomogeneous, and no evidence of focal abnormalities within the fusion mass. This uptake is probably due to widespread new bone formation. Uptake within the vertebral bodies or apophyseal joints at the fusion levels may be seen and are more common with posterior fusions. These findings may be due to motion within the fusion, despite the absence of pseudoarthrosis. Due to the alterations in impact loading following fusion, focal uptake in the vertebral bodies and apophyseal joints in the free motion segments adjacent to the fusion are commonly seen: 45% of patients following lateral fusion and 87% of patients following posterior fusion (posterior fusion has been shown to produce greater stress in the free segments adjacent to the fusion). A pronounced focal increase in uptake within the fusion mass more than 1 year post-op is highly suspicious for pseudoarthrosis. Generally, significant healing of the fusion is complete by 6 months, but as at other sites of surgery, scintigraphy may remain positive for up to 2 years. As a general rule if healing is taking place, the activity seen within the fusion should decrease in size and intensity. Focal abnormalities within the spinal fusion mass have also been identified in asymptomatic patients, and may represent painless pseudoarthrosis. Late pseudoarthrosis (over 4 years old) may not demonstrate increased tracer localization and this may be related to decreased osteoblastic activity. These lesions, although present, may not be clinically significant as they do not alter skeletal metabolic activity. Asymmetric SI joint activity is also noted frequently in patients that have undergone spinal fusion (up to 75% of cases), with the side of the bone graft harvesting showing less activity than the contralateral side. Increase SI joint uptake can also be seen and may be related to alterations in spinal mechanics [1]. Scintigraphy has a sensitivity of 78% and a specificity of 83% in the detection of pseudoarthrosis, which is superior to plain radiography (43% and 50%, respectively). [1-5]
Bone Grafts:
Bone scintigraphy can also be used to evaluate whether a bone graft is viable or non-viable. Vascular patency to the graft is characterized by normal or diffusely increased tracer uptake throughout the graft. Focally increased uptake may be seen at the osteotomy sites. When grafts have lost their vascularity they do not concentrate tracer and will appear as photon deficient areas on delayed bone images with reduced flow on the perfusion study. It is important to remember that the scan is most useful for predicting vascular patency and osseous metabolic activity ONLY when the scan is performed within one week of surgery. Scans performed later may be incorrectly interpreted as showing graft viability because of "periosteal creep" (i.e.: new bone deposition on a non-viable graft). Additionally, although tracer uptake is equated with graft viability, this finding has also been described in patients with osteoradionecrosis- a serious complication of radiotherapy for head and neck tumors. Consequently, the presence of increased uptake in patients who have undergone radiotherapy after mandibular reconstruction cannot be automatically equated with graft viability [1].
Joint Prosthesis Imaging:
By 10 years after implantation, 50% of prostheses demonstrate radiographic evidence of loosening and 30% require revision [15]. Infection is a serious complication of joint replacement and can occur in 1% to 4% of patients for hip prostheses (the rate of infection following revsion surgery is somewhat higher [15]). About one-third of infections develop within 3 months, another third within one year, and the remainder develop more than 1 year after surgery [15]. Staphylococcus epidermidis and S aureus are the most common organisms [15]. Septic prostheses require resection arthroplasty, a long course of antibiotics, and a lengthy hospitalization. In aseptic loosening, the patient typically undergoes a single stage revision arthroplasty [15]. Differentiation of aseptic loosening from infection is therefore vitally important [15]. Joint aspiration suffers from large numbers of false positive and false negative results [15]. Plain film findings are usually normal (50%) in patients with septic hip prosthesis. Non-focal periprosthetic bone resorption more than 2mm wide is non-specific and can also be seen in loosening.
Uptake at the distal tip of the prosthesis is not commonly seen (only about 10% of patients). If identified, it is typically mild. Activity greater than the intensity of the iliac crest seen only on delayed images (i.e.: normal flow and blood pool) is strongly indicative of loosening. If the uptake about the acetabular component of the prosthesis exceeds that of the activity of the iliac crest, this is also indicative of loosening.
Conventional bone scintigraphy has a sensitivity of 65%, specificity of 70%, and an accuracy of 50-70% [15] for the diagnosis of infected prosthesis. Classically, there is a focal abnormality identified about the prosthesis with loosening, and diffusely increased periprosthetic activity associated with infection [15]. Unfortunately, such findings are non-specific and there is considerable overlap between infection and loosening [15]. False positive scans can occur due to dystrophic ossification, periprosthetic granulomas, the altered distribution of red marrow, damage of the polyethylene surface of the prosthesis, and metallosis. None-the-less, bone scintigraphy is useful as a screening test because a normal scan essentially excludes a prosthetic complication [15].
Oswald et al. reported on the bone scan findings in uncomplicated porous coated hip arthroplasties [6]. Increased blood flow or focal blood pool abnormality are only rarely seen in uncomplicated prostheses. Diffusely increased blood pool activity within the muscle groups of the ipsilateral leg is seen in up to 40% of patients at some time during their post-operative course and can be seen as late as 24 months post operatively. This may be related to increase muscle usage in patients who were previously less active. Delayed activity at the distal tip of the prosthesis can be seen in all porous prosthesis at some time. Even 2 years post-op, activity could still be identified medially (15%), distally (70%), and laterally (50%). Activity in the medial segment was always less than or equal to lateral segment activity. Analysis of intensity over time demonstrated a clear trend toward stable or decreasing activity within the medial segment. Although this trend is generally observed in the distal and lateral segments as well, activity in these areas may also be noted to increase over time. This activity was not indicative of prosthetic loosening. Increased tracer activity can also be identified about the acetabular component of the prosthesis (present in up to 76% of patients even after two years) [13]. In uncomplicated prostheses, acetabular activity generally remains stable or decreases in intensity over time [13].
Persistently increased uptake of Tc-MDP in heterotopic bone occurs in most patients with hip prostheses. This is in contradistinction to Tc-MDP uptake in heterotopic bone in paraplegic patients which decreases as the bone matures.
In-111-WBC uptake was present at the prosthesis tip in 80% of patients at some time, and still present 2 years post-operatively in 50% of patients. Two distinct patterns of WBC uptake were identified: Focal (55%) or diffuse linear (45%) activity within the marrow space below the tip of the prosthesis. This uptake demonstrated a clear trend toward becoming stable or decreasing over time in 90% of patients. In 111-WBC activity was also usually less than (32-60%) or equal to (17%) the activity within the iliac crest. Abnormalities with activity greater than that in the iliac crest should be considered suspicious for infection. In comparison to the Tc-MDP scan, the intensity of In 111-WBC uptake at the tip of the prosthesis was less than or equal to the Tc-MDP uptake in 85% of patients (using the iliac crest as a reference). In-111 WBC accumulation also occurs about the acetabular component of the uncomplicated prosthesis in up to 92% of cases and can still be seen even after two years [13]. The activity is always less intense or equal to activity in the iliac crest and typically remains stable or decreases in intensity over time [13].
Mild WBC accumulation can be seen within heterotopic bone and this should not be confused with the presence of infection. Inguinal nodal activity may also be seen- the lateral view is helpful in demonstrating that the abnormality is anterior to the hip. Additionally, patients with heterotopic bone or inguinal nodal activity typically do not demonstrate a flow or blood pool abnormality on their bone scan. On average, combined In-111 WBC-bone scintigraphy imaging has a sensitivity of about 67%, and a specificity of 78% for the detection of prosthetic infection.
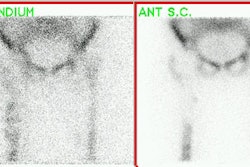
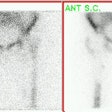
However, in leukocyte imaging it is neither the presence nor the intensity of labeled leukocyte activity, but rather the relationship of such uptake to bone marrow activity that is important [15]. Unexpected ectopic bone marrow may occur in the appendicular skeleton after trauma and surgical interventions such as joint prosthesis surgery [16]. Non-specific In-111 WBC accumulation within areas of ectopic bone marrow can be misinterpreted as infection [15]. The addition of Tc-sulfur colloid (Tc-SC) imaging can aid in evaluation of suspected prostetic hip infection by demonstrating normal bone marrow distribution. An sptially incongruent pattern of Tc-SC and In-111 WBC tracer activity should be considered consistent with infection [15]. When the distributions of the two tracers are spatial congruent, the exam is negative for infection [15]. Combined marrow scintigraphy using In-111 WBC's and Tc-sulfur colloid provides the best results with sensitivity, specificity, and accuracy greater than 90% [1,15].
Sequential bone-gallium imaging can also be used to evaluate for prosthesis infection, but the overall accuracy (70-80%) is less than that of combined In-111 WBC - Tc-Marrow scintigraphy [15]. For the gallium exam, the study is negative for infection when the gallium scan is normal, regardless of the bone scan findings, or when the spatial distributions of the two tracers are congruent and the intensity of gallium uptake is less than that of the bone tracer [15]. The exam is positive for infection when the distribution of the tow tracers are spatial incongruent or when their distributions are spatial congruent, but the intensity of gallium uptake exceeds that of the bone agent [15]. The images are quivocal for infection when the distribtuions of the two tracers are spatial congruent and the intensity of uptakes are similar [15].
Hip Prosthesis Loosening:
Plain film findings which support porous prosthesis loosening include finding a lucent line wider than 2mm adjacent to 3 of the 7 regions about the prosthesis. These findings are felt to represent mechanical loosening. Aggressive granulomatosis (granulomatous pseudotumor) represents a foreign body reaction to the cement or prosthesis itself, and is now also recognized as a frequent cause of prosthetic failure. It appears as well circumscribed areas of radiolucency adjacent to the prosthesis, which do not conform to the shape of the prosthesis and can progress over time. Both of these findings, however, have also been described in association with infection. None-the-less, serial plain film radiographs have sensitivities of 95% and 100% for the detection of loosening of the acetabular and femoral components (respectively) [14].
On bone scintigraphy, focal abnormalities in tow or more zones or intense uptake in one zone about the prosthesis is indicative of loosening [14]. For acetabular loosening, the sensitivity of bone scanning is 97%, and the specificity is 95% (Accuracy 90%) [14]. For femoral component loosening the bone scan sensitivity is 85%, and the specificity 100% (Accuracy 89%) [14]. Therefore, bone scan does not always provide additional information with regard to loosening of the hip arthroplasty compared to serial plain film evaluation [14]. Bone scan is probably most useful when the radiographs are inconclusive with regard to loosening [14]. In general, if the plain radiographs do not show loosening, an aspiration of the hip is negative, and there is no increased activity on a bone scan, then the hip arthroplasty is neither loose nor infected [14].
The incidence of infection after TKA has ranged from 1 to 2% [7,15]. Aspiration of the knee is helpful, but negative results do not exclude a deep infection [7]. It is even more difficult to assess total knee replacements, because 60% of the femoral components and 90% of the tibial components demonstrate increased periprosthetic activity more than 1 year after surgery in asymptomatic patients [1,8]. White blood cell imaging has a sensitivity, specificity, and accuracy of approximately 85% in the diagnosis of infected knee prosthesis [7]. False positive exams have been reported in association with active rheumatoid arthritis and with massive osteolysis of the adjacent femur and tibia [7].
REFERENCES:
(1) Semin Nucl Med 1995; Palestro CJ. Radionuclide imaging after skeletal interventional procedures. 25: 3-14
(2) J Nucl Med 1993; Collier BD, et al. Bone scintigraphy: Part 2. Orthopedic bone scanning. 34(12):2241-6. Review.
(3) J Nucl Med 1989; Lusins JO, et al. Bone SPECT in patients with persistent back pain after lumbar spine surgery. 30: 490-96
(4) J Nucl Med 1994; Even-Sapir E, et al. Assessment of painful late effects of lumbar spinal fusion with SPECT. 35(3):416-22.
(5) Skeletal Radiol 1987; Slizofski WJ, et al. Painful pseudarthrosis following lumbar spinal fusion: detection by combined SPECT and planar bone scintigraphy. 16(2):136-41
(6) J Nucl Med 1989; Oswald SJ, et al. Three-phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. 30(8):1321-31.
(7) Clinical Orthopaedics and Related Research 1990; Rand JA, Brown ML. The value of indium 111 leukocyte scanning in the evaluaiton of painful or infected total knee arthroplasties. 259: 179-182
(8) Nuclear Medicine Annual 1994, Palestro CJ. Musculoskeletal infection. Ed. Freeman LM. Raven Press, Ltd. New York. 91-119
(9) Radiology 1988; Magnuson JE, et al. In-111-labeled leukocyte scintigraphy in suspected orthopedic prosthesis infection: Comparison with other imaging modalities. 168: 235-239
(10) British Journal of Radiology1992; Miles BKA, et al. Scintigraphic abnormalities in patients with painful hip replacements treated conservatively. 65: 491-494
(11) British Journal of Radiology1992; Copping BC. The role of 99m-Tc-HMPAO white cell imaging in suspected orthopaedic infection. 65: 309-312
(13) J Nucl Med 1990; Oswald SG, et al. The acetabulum: A prospective study of three-phase bone and indium white blood cell scintigraphy following porous-coated hip arthroplasty. 31: 274-280
(14) J Bone Joint Surg [Br] 1993; Lieberman JR, et al. Evaluation of painful hip arthroplasties. Are technetium scans necessary? 75-B: 475-78
(15) Radiographics 2001; Love C, et al. Role of nuclear medicine in diagnosis of the infected joint replacement. 21: 122901238
(16) J Nucl Med 1998; Kaim A, et al. Ectopic hematopoietic bone marrow in the appendicular skeleton after trauma. 39: 1980-1983