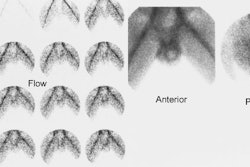
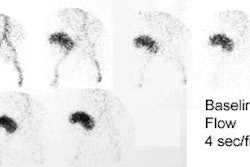
[131I] 6-beta-iodomethyl-19-norcholesterol: NP-59
Pharmacology and Physiology
The precursor molecule from which all adrenocortical steroid hormones are synthesized is cholesterol [5]. NP-59 is a cholesterol analog which will localize to adrenal cortical lesions such as adrenal adenomas. It is most useful in the evaluation of incidentally detected adrenal masses and hyperaldosteronism. The examination is less useful in the evaluation of Cushings syndrome, which is most commonly due to bilateral adrenal hyperplasia.
NP-59 is transported in the circulation like native cholesterol, bound to low-density lipoproteins. These will bind to specific low-density lipoprotein receptors on adrenocortical cells, and also within the liver. After binding to adrenocortical cells the NP-59 is internalized and esterified, but it is not further metabolized [1]. In the liver the agent is metabolically converted into bile salts (as with ordinary cholesterol) and excreted in the bile.
The uptake of NP-59 is influenced by adrenocortical secretogogues such as adrenocorticotropic hormone (ACTH) and Angiotensin II which will increase uptake of the tracer [1]. Adrenal uptake of the agent is 50% dependent on ACTH and 10% dependent on angiotensin II. Dexamethasone and exogenous steroids will have the opposite effect, and reduce tracer uptake [1]. Uptake of the tracer is also inversely related to circulating levels of cholesterol, and its uptake may be severely reduced in patients with marked hypercholesterolemia due to competitive inhibition at the binding sites [1].
The enterohepatic excretion of NP-59 and its metabolites may result in significant bowel activity which can obscure the adrenal glands. Lowering of background activity can be aided by the use of laxatives to speed bowel excretion. The gallbladder may also be visualized and can be confused with the right adrenal gland (A lateral image will show that the GB lies anteriorly.) The typical dose is 1mCi/1.73m2 (Max. dose of 2mCi). To block thyroid uptake of free 131I, Lugol's solution or SSKI (Saturated potassium iodide solution) is given to protect the thyroid as there is significant in vivo deiodination of the agent. The critical organ is the adrenal gland which receives about 26 rad/mCi.
Non-suppressed exam
Indicated for the evaluation of Cushings syndrome, an incidental adrenal mass, or adrenal remnant. The normal adrenal glands are usually visualized on the unsuppressed exam. The right adrenal may appear more intense on posterior images due to its closer proximity to the detector.
Dexamethasone suppression exam
The dexamethasone suppression exam is indicated for the evaluation of primary aldosteronism or hyperandrogenism. Suppression helps to improve the specificity and diagnostic accuracy of the NP-59 exam in the evaluation of primary aldosteronism because aldosterone hypersecretion does not result in suppression of pituitary ACTH secretion. Thus, the adrenal glands can be visualized normally on routine imaging in these patients, making detection of an adenoma more difficult.
For this exam, dexamethasone (4-8 mg daily in divided doses for 3-7 days and continued through the exam) is used to suppress the normal adrenal cortex in order to accentuate any abnormality. Images are performed 3, 4, and 5 days after injection of the tracer. Breakthrough visualization of the normal adrenal glands can be expected after 5 days, but an adenoma (unilateral activity) or hyperplasia (bilateral activity) is usually evident by day 3.
Medications which stimulate plasma renin activity must be avoided for 4 weeks prior to the examination if high sensitivity for adenoma detection is to be maintained. Such drugs include: diuretics, angiotensin converting enzyme inhibitors, calcium channel blockers, and spironolactone.
Disorders Imaged with NP-59
Cushing's Syndrome
Clinical Presentation
Cushing's syndrome is the result of excess glucocorticoid. It is characterized by truncal obesity, a moon facies, capillary fragility, dementia (depression), osteoporosis, and immunodeficiency.
Etiologies of Cushings Syndrome with Excess ACTH (80% of cases of Cushings syndrome):
Cushings Disease
Cushings disease is due to pituitary overproduction of ACTH (secondary to a pituitary
adenoma).
Ectopic ACTH
Lung cancer is a type of cancer than may be associated with ectopic ACTH production,
particularly small cell carcinoma.
Exogenous ACTH administration
NP-59 imaging is generally not useful in the setting of excess ACTH since the disease process is located outside the adrenal gland. Increased ACTH produces bilateral adrenal hyperplasia of the inner adrenal cortex or zona fasciculata in the vast majority (up to 85%) of cases of Cushings syndrome. These patients have elevated ACTH levels, as well as elevated levels of cortisol and corticosteroid metabolites.
The adrenal glands can undergo 2 types of hyperplasia:
- Smooth hyperplasia, which is more commonly seen, or
- Cortical nodular hyperplasia, which is less common.
Nodular hyperplasia can be micronodular (in which the glands appear normal on CT), or macronodular, which may occasionally mimic a corticaladenoma.
Bilateral, symmetric visualization of the adrenals on the NP-59 exam in a patient with biochemically confirmed glucocorticoid (ACTH) excess is invariably due to adrenal hyperplasia (In the setting of excess ACTH the NP-59 scan typically demonstrates bilateral, symmetric increased accumulation of the tracer). Mild adrenal asymmetry, however, can be seen in the setting of cortical nodular hyperplasia. In one recent study (Surgery, 1995 p986) NP-59 scintigraphy was superior to CT/MRI in predicting unilaterality versus bilaterality in Cushings syndrome due to primary adrenal disease.
A rare cause of Cushings syndrome is primary pigmented nodular adrenal cortical hyperplasia which is usually seen in children or young adults. These patients often have short stature, delayed puberty, or severe osteoporosis, and may be associated with Carney's disease (myxomas of cardiac, breast, and soft tissue; giant calcifying Sertoli cell tumors of the testes; and lentigenes of the skin and oral mucosa). The adrenals may have a characteristic "string-of-beads" appearance in these patients.
Etiologies of Cushings Syndrome with Suppressed ACTH or ACTH Independent (20% of cases of Cushings syndrome):
Adrenal adenoma
Accounts for the majority of cases of ACTH independent Cushings syndrome (90%). On the
NP-59 exam there is typically unilateral increased tracer accumulation with suppression
(non-visualization) of the opposite gland. On CT, the opposite gland typically has a
normal appearance, although atrophy of the contralateral gland may rarely be seen.
Adrenal carcinoma
Accounts for 5-10% of cases of ACTH-independent Cushings syndrome in adults (carcinoma is
etiology of ACTH independent Cushings in up to 50% of children). About 50% of adrenal
carcinomas are functioning in which case the NP-59 exam demonstrates bilateral
non-visualization of the adrenals due to the carcinoma on one side, and suppression of the
contralateral adrenal. Markedly elevated cholesterol levels and/or prior exogenous
glucocorticoid administration may also rarely result in bilateral non-visualization of the
adrenal glands.
ACTH independent hyperplasia
Bilateral, asymmetric uptake greater than 50% suggests ACTH independent hyperplasia
(autonomous, bilateral nodular hyperplasia). In these patients ACTH levels are usually
low, while urinary and plasma cortisol levels are elevated.
Exogenous glucocorticoids
Correlative Examinations in the Evaluation of Cushings Syndrome
Petrosal sinus sampling is the most accurate test available to distinguish between Cushings disease and the ectopic ACTH syndrome. Pituitary venous drainage is represented in blood samples obtained from the inferior petrosal sinuses. A ratio of ACTH concentrations (inferior petrosal sinus sample: peripheral vein sample) of greater than 2.0, or greater than 3.0 following CRH stimulation, is indicative of Cushing's disease. A ratio between the two sinuses of over 1.4 will also correctly localize a pituitary microadenoma in 70% of patients.
Primary hyperaldosteronism: Conn's Syndrome
Clinical Presentation
Aldosterone is the principal mineralocorticoid secreted by the outer adrenal cortex, the zona glomerulosa. (A useful mnemonic for memorizing the hormone products and relative locations of the zona glomerulosa, fasciculata, and reticularis of the adrenal cortex is "G -> F -> R : Salt -> sugar -> sex : The deeper you go, the better it gets [Sorry for the 'sexist' remark, but it may help someone remember]..)
Patients present with elevated plasma or urinary aldosterone levels, hypertension, hypokalemia, metabolic alkalosis, and decreased renin levels. Primary hyperaldosteronism occurs most commonly secondary to an autonomous adrenocortical adenoma or aldosteronoma (80% of cases). Bilateral adrenal hyperplasia accounts for the other 20% of cases. Clinically, patients with adrenal hyperplasia as the etiology may demonstrate increased serum aldosterone levels during upright posturing after prolonged recumbence, while levels decline or are unchanged in patients with an adenoma. Adrenal carcinoma is the etiology in less than 1% of cases. Surgery is effective only for adrenal adenomas; bilateral adrenalectomy for hyperplasia is ineffective and medical therapy is indicated.
Radiographic Findings
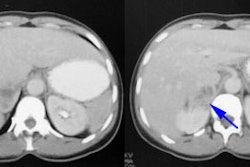
On CT an adenoma appears as a small, water density adrenal mass. Unfortunately, aldosteronomas are usually very small and the detection rate for these lesions by CT is poor (about 60%). Additionally, bilateral nodularity is not synonymous with hyperplasia, and adrenal venous sampling is required to detect the presence of a surgically correctable adrenal adenoma in these patients.
When using adrenal vein sampling, the aldosterone-cortisol ratio in the adrenal vein to the inferior vena cava will be greater than 1.5 if there is an adrenal adenoma (normally, this ratio is near unity). Hyperplasia results in values above 1.5 for both sides. Adrenal venous sampling has an accuracy of 95 to 100%, but successful bilateral sampling is achieved in only 75% of cases because of difficulties in sampling the right adrenal vein.
Scintigraphic Examination
Prior to the exam, patients must be removed from medications and antihypertensives that can affect NP59 accumulation including glucocorticoids, diuretics, beta blockers, spironolactone, calcium channel blockers, or alpha-blockers [7]. The NP-59 exam has a sensitivity of 85% and a specificity of 95% in the detection of adrenal adenomas in primary hyperaldosteronism. The sensitivity and specificity of NP-59 scintigraphy is increased when dexamethasone suppression is used to amplify the differences between the secretory capacity of the ACTH dependent inner adrenal cortex (zona fasciculata and zona reticularis) from the renin-angiotensin dependent outer cortex (zona glomerulosa). An adrenal adenoma demonstrates early unilateral activity, while hyperplasia appears as bilateral early adrenal activity. Breakthrough adrenal activity is usually not visualized until day 5. Because the resolution of planar imaging is limited, SPECT/CT can aid in lesion detection [7].
Adrenal Hyperandrogenism
Adrenogenital syndrome
This uncommon disorder is due to an inborn error in the enzyme 11-beta or 22-hydroxylase, which are essential in the production of cortisol and aldosterone. This defect leads to symmetric adrenal hyperplasia and is usually seen in newborns and infants. In this setting, the dexamethasone suppression NP-59 exam will demonstrate bilateral, symmetric early (before 5 days) adrenal visualization.
Other causes
Adrenal adenomas can also cause adrenogenital syndrome and these patients generally present at a later age. The NP-59 exam will lateralize to the side of the adenoma.
The absence of tracer accumulation suggests another etiology such as a masculinizing or feminizing tumor. Ovarian accumulation has been identified in patients with androgen-secreting ovarian tumors (arrhenoblastomas) and other dysfunctional ovarian states.
Non-hyperfunctioning Adrenal Adenoma
Non-hyperfunctioning adrenal adenomas (i.e.: there is no biochemical evidence of adrenal hyperfunction) are detected in about 8% of patients at autopsy, and in approximately 2% of patients undergoing abdominal CT scanning. Even in patients with lung cancer, an adrenal mass is more likely to be an adenoma than a metastasis [2].
CT features of a benign adenoma include a smooth contour, smaller than 5 cm in size, and an attenuation value of zero or less (values between 0 and 10 indicate a high probability of a benign lesion, but values over 10 may be benign or malignant and further evaluation is warranted).
On MRI, most adenomas are iso- or hypointense to the liver on T2-weighted images and demonstrate a high fat (lipid) content on chemical shift images. Adenomas demonstrate only mild enhancement with gadolinium and quickly washout, unlike metastases which tend to have very prominent enhancement and slow washout. Most adrenal adenomas arise from the zona fasciculata; however, those which arise from the zona reticularis (10 to 30%) contain lipid depleted cells and can appear isodense to the liver on CT and hyperintense to the liver on T2 MRI images. Nonetheless, a hyperintense lesion on T2-weighted images is more commonly associated with adrenal carcinoma.
In asymptomatic adrenal tumors, size is also an important factor in determining malignancy and some suggest that lesions larger than 3 cm should be surgically removed. Fine needle aspiration biopsy of all adrenal lesions that cannot be classified as an adenoma on CT or MRI remains the most practical thing to do if a definitive diagnosis of metastatic disease is crucial to patient management. NP-59 scintigraphy may be useful in selected cases. [3]. NP-59 accumulation within the lesion indicates that the lesion is a benign adenoma (functioning, but non-hypersecretory) and no further workup is necessary. However, false positive examinations have been recorded with a pheochromocytoma and a myelolipoma [5]. These false positive exams may be the result of residual functional cortical tissue in the adrenal lesions [5].
Non-visualization of the contralateral normal gland can be seen and implies partial functioning autonomy of the adrenal mass despite overall normal adrenal function as defined by biochemical parameters which are within the normal range. Caution in interpretation should be used when the adrenal mass is less than 2 cm in diameter as a normal symmetric pattern of uptake can be identified. Absent uptake is more indicative of malignancy (met or carcinoma), but can be seen with a simple adenoma if there has been hemorrhage or calcification within the lesion. Sensitivity of the NP-59 exam is 71%, Specificity 100%, Negative Predictive Value 91%, and a Positive Predictive Value of 89-100%. [3,5].
A recent study in a small number of patients revealed that adrenal imaging with F-18 FDG had a diagnostic accuracy of 100% in the differentiation of benign from malignant incidentally detected adrenal masses [4,5].
REFERENCES:
(1) Radiologic Clinics of North America 1993; Sandler MP, et al. Radionuclides in
endocrine imaging. 31 (4): 909-921
(2) Radiology 1992;184:1-13
(3) J Nucl Med 1994; Falke TH, et al. Classification of silent adrenal masses: time to get practical. 35(7):1152-4. (No abstract available)
(4) Radiology 1995; Boland GW, et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. 194(1):131-4.
(5) J Nucl Med 2001; Maurea S, et al. The diagnostic role of radionuclide imaging in evaluation of patients with nonhypersecreting adrenal masses. 42: 884-892
(6) Semin Nucl Med 1995; Freitas JE. Adrenal cortical and medullary imaging. 25: 235-250
(7) J Nucl Med 2009; Yen RF, et al. 131I-6β-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. 50: 1631-1637