Renal and Urinary Tract Imaging
Radiopharmaceuticals
Tc-MAG3: (Mercaptoacetyltriglycine)
The typical dose of MAG3 in adults is 10 mCi (370 MBq). This dose
allows evaluation of the flow portion of the exam [8]. However, in
cases not including renal transplant evaluation, some authors feel
that the flow portion of the exam does not contribute to overall
exam interpretation and that the dose of MAG3 can be decreased by
50-90% (dose between 37-185 MBq) [8].
For children the dose is .1 mCi/ kg, minimum dose .5 mCi. Its radiochemical purity must be greater than 90%. In plasma between 66 to 90% (81% mean) of MAG3 is protein bound (which helps to prevent glomerular filtration).
MAG3 is highly protein bound and cleared almost exclusively by tubular secretion-- 98% is secreted in the proximal tubule and only 2% of the agent is filtered at the glomerulus. The agent is removed from the plasma primarily by organic anion transporter 1 located in the basolateral membrane of the proximal renal tubules [6]. The agent accumulates in the proximal tubular cell and is then transported into the tubular lumen via organic anion transporters on the apical membrane [6]. If the apical membrane transporter is impaired to a greater extent than the basolateral transporter, there will be retained parenchymal activity [6]. A small amount of liver activity is often noted. Increased liver activity is seen in patients with underlying renal failure (Creatinine > 2) and gallbladder activity (due to vicarious biliary excretion) may also be seen in these patients [6]. The extraction fraction of MAG3 is between 40 to 50% (more than twice that of DTPA) and the average renal clearance is between 320 ml/min.- which is much higher than the normal GFR (125 ml/min.) and decreases by approximately 1% per year after age 40 years [6].
Although renal clearance is approximately 56% of the renal
clearance of orthoiodohippurate (600 ml/min.), OIH and MAG3 are
excreted from the body at essentially the same rate. The high
protein binding of MAG3 (80% versus 60% for OIH) and lack of red
blood cell entry (as with OIH) means that more MAG3 is available
within the intravascular compartment for extraction (i.e.: there
is less third spacing). Image quality with MAG3 is also superior
to DTPA, particularly in patients with impaired renal function,
because MAG3 has a higher renal clearance, resulting in good
kidney to background ratios. The relatively high extraction
efficiency and the small distribution space of MAG3 result in fast
accumulation of high intensity activity in the cortex and
subsequently the collecting system.In patients with normal renal
function, over 95% of the MAG3 dose leaves the body by 3 hours
[6].
The critical organ is the bladder wall.
Tc-DTPA (Diethylenetriamine-pentacetic acid)
The typical dose of Tc-DTPA in adults is 10-20 mCi. For children the dose is .1 mCi/ kg, minimum dose .5 mCi. The agent can be used to evaluate renal perfusion, glomerular filtration, and for renal and urinary tract imaging. DTPA is the only renal radiopharmaceutical available for routine imaging that is cleared entirely by glomerular filtration (similar to inulin) [6]. Consequently, it is the only agent that can be used to measure GFR [6]. Unfortunately, the renal extraction efficiency (percentage of tracer extracted with each pass through the kidney) is low (20%) and this results in a low target-to-background ratio [6]. About 90% of the injected dose is in the urine by 4 hours. Because a small but variable amount (0 to 9%, depending on the kit formulation) of the agent is plasma protein bound, there is a slight underestimation of the true GFR (Calculated GFR must be corrected for this bound fraction).
See ICRP 53, p186, for detailed dosimetry information. The critical organ is the bladder wall.
131I/ 123I-Hippuran (Orthoiodohippurate)
Orthoiodohippurate imaging agents are no longer commercially available in the United States [6]. The typical dose of 131I-Hippuran used in adults is 150-300 uCi. For children the dose is 3 uCi/kg, minimum dose 25 uCi. Hippuran is used for evaluation of tubular secretion (excretory function) because it behaves similarly to PAH. About 70% is protein bound in plasma, and 5% diffuses into the red cell. 80% is eliminated by tubular secretion in the proximal tubules, and 20% by glomerular filtration. There is 90% first-pass clearance (total extraction efficiency is high (70-90%), which leads to a high kidney to background ratio) and maximum renal activity is seen within 5 minutes. The renal clearance of OIH is approximately500-600 ml/min [6]. The relative renal uptake of OIH is proportional to the relative effective renal plasma flow (ERPF). Approximately 70% of the administered dose is in the urine by 30 minutes after injection (similar to MAG3).
Because it has a higher renal extraction efficiency than DTPA, hippuran provides better visualization of the kidneys in patients with markedly impaired renal function. In acute renal failure, non-visualization of the kidneys with hippuran is a poor prognostic indicator. The critical organ is the thyroid. The radiation dose to the thyroid can be minimized by giving perchlorate or Lugol's prior to the study.
See ICRP 53, p310, for detailed dosimetry information. The critical organ is the bladder wall, except in patients with obstruction or cortical retention may become very high. In severe cases, kidney doses will be several hundred rem.
Tc-DMSA (2,3-dimercaptosuccinic acid)
The typical dose in adults is 5 mCi (Minimum dose 300 to 350 uCi). For children the dose is .05 mCi/ kg, minimum dose .3 mCi. DMSA is used for high resolution imaging of the renal cortex [6]. The agent is slowly cleared from the blood as a result of its high protein binding. Only about 4% is extracted per pass. DMSA localizes to the renal cortex by binding to sulfhydryl groups in proximal renal tubules. (40 to 50% of the injected dose is present in the renal cortex 2 hours after injection). Although the agent does undergo glomerular filtration (4 to 8% by 1 hour, and about 30% by 4 hours), the renal collecting system is usually not visualized. Peak renal activity is seen 4 to 6 hours post injection, but images are usually acquired before this time. Studies suggest that DMSA is bound to alpha-1-microglobulin and that the DMSA-microglobulin coomplex is then filtered by the glomerulus and accumulates in the kidneys via meglin/tubulin mediated endocytosis from the glomerular filtrate [6]. Renal uptake of DMSA is therefore dependent on renal blood flow, glomerular filtration, and proximal tubule receptor mediated endocytosis [6].
Acidification of the urine will increase excretion of the agent; as a result patients with renal tubular acidosis often demonstrate poor renal visualization.
The critical organ is the kidneys.
Tc-Glucoheptonate
The typical dose in adults is 15 mCi. For children the dose is 0.1 mCi/ kg, minimum dose 0.5 mCi. Glucoheptonate has both cortical uptake and glomerular filtration. A large portion is cleared by glomerular filtration and rapidly excreted (80-90% cleared via urine), but a significant amount remains in the proximal tubular cells which permits cortical imaging (10-20% is retained in renal tubular cells 1 to 2 hours after injection). Glucoheptonate has an extraction efficiency of about 20%. Hepatic excretion can be seen in patients with marked renal dysfunction.
See ICRP 53, p194, for detailed dosimetry information. The critical organ is the bladder wall.
Renal Perfusion/Function Exam Findings
Renal activity can be demonstrated when function is as low as 3%
of normal.Patients should arrive well hydrated for their renal
scan and should drink two large glasses of water just before
arrival [6]. Diclofenac is a non-steriodal anti-inflammatory drug
that blocks the production of prostaglandins and has been shown to
inhibit spontaneous ureteric contraction, prolonging transit time
and delaying the time to peak activity on MAG3 scans [6].
A complete discussion of the renal scan should address each of the following areas:
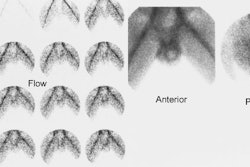
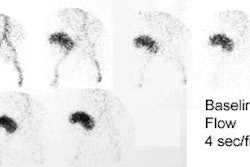
1- Perfusion
Flow activity should reach the kidneys about 1-3 sec after the bolus in the abdominal aorta passes the renal arteries. When using DTPA the slope of the renal flow curve should be similar to that of the aorta. After reaching a peak value, the renal curve decreases, reflecting the low extraction efficiency (20%) of the agent. With MAG3, the renal flow curve will reach a certain peak level, but not decline. Instead, it continues to slowly increase, reflecting the excellent extraction efficiency of MAG3.
Some experts believe the flow study adds little to the routine renogram, and need not be performed, except in cases of acute renal failure or tranplant studies [1].
2- Extraction (Parenchymal activity)
The intensity of the peak parenchymal activity should approach
that of the aorta. Parenchymal activity should peak by 3-5
minutes, and then rapidly decline. Dehydrated patients will
demonstrate peak parenchymal activity slightly later and may have
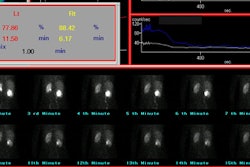
delayed clearance of the tracer. The differential or relative
renal function of each kidney can be calculated by determining the
accumulation of tracer in each kidney between 1 and 2 or 2.5
minutes after injection of MAG3 and between 2 and 3 minutes for
DTPA.
An inferior placed background ROIU undercorrects background for
both MAG3 and DTPA studies [7]. For the calculation of relative
renal uptake, a perirenal background ROI normalized to the kidney
ROI is preferred over a background region placed inferior to the
kidney [6,7] (typically a C-shaped or elliptical background ROI).
During the extraction period, all the activity should be confined
to the renal vasculature and functioning renal parenchyma
(excluding non-extracted blood pool tracer) with little activity
within the collecting system. Differences in renal depth can
affect differential function determination. A geometric mean is
generally a more reliable indicator of differential function than
the count ratios from posterior images alone. A normal posterior
differential function is within 56%/44%; a differential uptake of
greater than 60%/40% is likely abnormal.
In an animal model, certain NSAID can affect renal function and the renogram curves- diclofenac is used in the treatment of renal colic and can result in a shift of the renogram curves to the right- especially with DTPA imaging.
3- Excretion (From parenchyma into collecting system)
Collecting system activity is normally visualized between 3 to 5 minutes after injection. Activity should appear symmetric from side to side. Parenchymal MAG3 activity falls quickly and at 20 minutes there is usually less than 20% of the peak activity remaining in the kidney (18% +/- 6%)- this is referred to as the 20 min/max ration. If the patient is not dehydrated and the 20 min/max ratio exceeds 35% the kidney is likely abnormal [6].
4- Clearance
Activity should clear rapidly from the intrarenal collecting system to the bladder.
Renogram curves from each kidney should be nearly identical with
regard to shape and slope. Slight differences in height of the
curves may be due to normal variation in renal size or depth,
patient positioning, or differences in region of interest
placement.
MAG3 clearance can be estimated from the dose injected and the
amount of radioactivity in a single blood sample obtained
approximately 45 minutes following injection [6]. The GFR can be
estimated from the dose injected and the activity in one or
twoplasma samples obtained 1-4 hours after injection [6]. However,
plasma samplingh techniques have several sources of error- they
assume a normal volume of distribution; if the patient has
ascites, marked edema, or a large effusion, the tracer can diffuse
into these extrafluid spaces and plasma clearance will not provide
an accurate meausre of renal clearance [6].
Scintigraphic Findings in Renal Failure:
Acute renal failure is characterized by relative preservation of renal blood flow and extraction of tubular agents with a rising renogram curve (Retained cortical activity at 20 minutes (RCA) will be almost 100%). Extraction of glomerular agents in acute renal failure is low. The kidneys are usually of normal size.
In chronic renal failure there is severely and proportionately reduced renal blood flow and tracer extraction of both tubular and glomerular agents. The kidneys are often small in size. The renogram curve is typically flat with a low count plateau. In end-stage renal failure the kidneys are only faintly discernible from blood pool activity.
Cortical Necrosis
Cortical necrosis is a form of ATN. Patients present with oliguria or anuria. Pathologically, there is cortical ischemic necrosis. Etiologies of Cortical Necrosis include:
- Post-partum hemorrhage (abruptio placenta)- 50% of
cases
- Bacterial sepsis
- Shock
- Myocardial Infarction
- Burns
- Severe dehydration (children)
- Hyperacute transplant rejection
Radiographic Findings in Cortical Necrosis:
On IVP, early in the disease process there are enlarged, smooth kidneys with an absent or faint nephrogram. Later the scout film will show "Tram-line"cortical calcification (occurs subcapsular and at the corticomedullary junction) with no nephrogram or collecting system opacification.
On US, acutely there is decreased cortical echogenicity. Later, the cortex becomes hyperechoic with acoustic shadowing due to calcification.
Scintigraphic Findings in Cortical Necrosis
On scintigraphy there is poor to no renal perfusion and poor parenchymal tracer accumulation of tubular agents (little to no renogram). The kidney may appear as a photopenic region.
Determination of Kidney Function
The GFR indicates the amount of glomerular filtrate formed per minute (Normal = 125 ml/min.). Agents used to calculate the GFR must be freely filterable through the glomerulus and be neither secreted nor absorbed by the renal tubules. Inulin, EDTA, iodothalamate, and diatrizoate can all be used in GFR determinations. Regardless of the method of global GFR determination, individual renal GFR's are then calculated by multiplying the total GFR by their respective differential functions. At birth, the GFR is about 20% of the adult value, and reaches about 50% by 2 weeks of age.
Renal plasma flow represents the total perfusion to the kidney, only part of which is accounted for by the GFR. 'Effective' renal plasma flow is calculated using PAH in the laboratory (PAH slightly underestimates actual RPF because only 91% of the agent is extracted by the tubules during renal transit). The normal ERPF is approximately 600 ml/min. True renal plasma flow is about 650 ml/min. 131I OIH and Tc-MAG3 can also be used in the determination of ERPF.
Clinicians have relied upon various empiric schemes for estimating GFR which manipulate easily measured clinical parameters of age, weight, height, and serum and urinary creatinine, despite the well known inaccuracies of these methods. Since GFR can deteriorate to 1/2 its normal value before serum creatinine changes, creatinine values are dependent on adequate muscle mass, and creatinine is tubularly reabsorbed in severe renal failure, the inaccuracies of these methods areself evident.
Estimation of GFR and ERPF by the above radiopharmaceuticals has many theoretical advantages. Unfortunately, the literature is filled with a bewildering array of radionuclide techniques involving various combinations of gamma camera imaging, and blood and urine sampling, all of which involve significant and often considerable attention to detail, wet lab expertise, or specialized gamma cameracomputer processing algorithms. Accuracy of the techniques seems directly porportional to their difficulty. Considerable effort has been directed toward simplifying complex algorithms and lenghty and arduous (for the patient and practitioner) without significant loss of accuracy. A recent consensus from the Radionuclides in Nephrology Group, Committee on Renal Clearance [3] offers recommendations for specific techniques in various clinical situations. Conspicuous is a lack of endorsement of gamma camera techniques, which, despite their reproducibility, are generally very inaccurate in moderate to severe renal dysfunction. The relative merits of various methods, especially in the pediatric population, is hotly debated.
REFERENCES:
(1) J Nucl Med 1991; Blaufox MD. Procedures of choice in renal nuclear medicine. 32:1301-9
(2) Semin Nuc Med, 1992; Conway JJ. "Well-tempered" diuresis renography: its historical development, physiological and technical pitfalls, and standardized technique protocol. 22: 74-84
(3) J Nucl Med 1996; Blaufox MD, et al. Report of the Radionuclides in Nephrourology Committee on renal clearance. 37(11): 1883-90
(4) Nucl Med Annual 1991; Dubovsky EV, Russell CD. 99mTc-MAG3: The multipurpose renal radiopharmaceutical. Ed. Freeman LM. Raven Press, Ltd. New York. 1-35
(5) Clin Nucl Med 1993; Dogan AS, Kirchner PT. Hepatobiliary
excretion of MAG3. Simulation of a urinary leak. 18: 746-750
(6) J Nucl Med 2014; Taylor AT. Radionuclides in nephrourology,
part 1: radiopharmaceuticals, quality control, and quantitative
indices. 55: 608-615
(7) J Nucl Med 2014; Taylor AT. Radionuclides in nephrology, part
2: pitfalls and diagnostic applications. 55: 786-79
(8) J Nucl Med 2017; Taylor AT, et al. 99mTc-MAG3:
image wisely. 284: 200-209