Society of Nuclear Medicine Procedure Guideline for
Renal Cortical Scintigraphy in Children
Authors: Gerald A. Mandell, MD (DuPont Hospital for Children, Wilmington, DE); Douglas F.
Eggli, MD (Pennsylvania State University/Milton S. Hershey Medical Center, Hershey, PA);
David L. Gilday, MD (Hospital For Sick Children, Toronto, Ontario, Canada); Sydney Heyman,
MD (Children’s Hospital of Philadelphia, Philadelphia, PA); Joe C. Leonard, MD (Oklahoma
Children’s Memorial Hospital, Oklahoma City, OK); John H. Miller, MD (Children’s Hospital Los
Angeles, Los Angeles, CA); Helen R. Nadel, MD (Children’s Hospital, British Columbia,
Vancouver, Canada); and S. Ted Treves, MD (Children’s Hospital, Boston, MA).
I. Purpose
The purpose of this guideline is to assist nuclear medicine practitioners in recommending, performing, interpreting, and reporting the results of renal cortical scintigraphy in children.
II. Background Information and Definitions
Renal cortical scintigraphy is used for the detection of the cortical defects of acute pyelonephritis and scarring related to chronic pyelonephritis. Cortical scintigraphy is able to detect twice as many defects as ultrasound and four times as many defects as intravenous urography. The loss of function associated with acute pyelonephritis, when detected early and satisfactorily treated, can be reversed without scar formation. The sequelae of renal infection can be monitored by follow-up cortical scintigraphy. Computed tomography has a similar sensitivity and specificity to cortical scanning for the detection of acute pyelonephritis, but adds to the risk of contrast reaction and has a higher radiation exposure. Magnetic resonance imaging is a promising but expensive non-ionizing method of visualizing pyelonephritis.
The commonly used clinical and laboratory parameters are not reliable for the diagnosis of acute pyelonephritis. The presenting symptoms can be varied and sometimes confusing. Patients may present with fever, flank pain or tenderness, malaise, irritability, leukocytosis, and bacteriuria, but there may be no definite indication of renal parenchymal infection. Neonates in particular present with nonspecific clinical findings. The higher grades of vesicoureteral reflux are generally associated with a greater risk for the development of pyelonephritis; however, acute pyelonephritis in the absence of vesicoureteral reflux is frequently seen.
III. Common Indications
A. Acute pyelonephritis
B. Renal scarring
C. Relative functioning renal mass
D. Solitary or ectopic renal tissue (e.g. pelvic kidney)
E. Horseshoe and pseudohorseshoe kidneys
F. Allergy to iodinated contrast agents
IV. Procedure
A. Patient Preparation
1. Preparation prior to arrival in the department
a. No preparation is necessary if sedation is not required for performance of the study. Some infants and young children will fall
asleep if sleep-deprived for several hr and fed just prior to the delayed imaging.
b. An informed consent, patient preparation, and presedation evaluation are necessary for administration of sedation.
i. Sedation may have to be administered for the performance of procedures which require the younger child or
uncooperative older child to remain motionless for a prolonged period of time.
ii. High-resolution pin-hole collimation and single photon emission computed tomography (SPECT) require prolonged
imaging times (30 min or more) and motion obscures the presence of defects in the cortex of the kidneys.
iii. Details of pediatric sedation can be referenced from Society of Nuclear Medicine Procedure Guideline for Pediatric
Sedation in Nuclear Medicine and the guidelines published by the American Academy of Pediatrics.
2. Preparation prior to injection of the radiopharmaceutical a. The procedure is explained to parents and to children old enough to understand.
b. Continual communication and reassurance with explanation of each step is essential for cooperation and successful intravenous injection of the radiopharmaceutical.
B. Information Pertinent to Performing the Procedure
1. A history of prior surgery to the urinary tract, congenital urinary abnormalities (duplex systems, ectopic renal tissue, renal fusion etc.), urinary tract obstruction, and question of mass lesion is important for accurate interpretation.
2. The review of available past radiographic, ultrasound and radionuclide studies adds to the accuracy of interpretation of the current study.
3. A history to evaluate for allergic reactions and previous complications to sedation.
C. Precautions
Patients with renal tubular acidosis show reduced tubular concentration of Tc-99m DMSA and increased excretion in the urine.
D. Radiopharmaceutical
1. Tc-99m dimercaptosuccinic acid (Tc-99m DMSA) is bound to proximal tubular cells with 40% to 65% of the injected dose present in the cortex 2 hr after the injection. The greater amount of activity in the cortex permits better resolution of cortical defects. DMSA is preferred in small infants.
2. Tc-99m glucoheptonate (Tc-99m GH) is partially concentrated and excreted in the urine and partially bound to the renal tubules with 10% to 20% of the injected dose present in the proximal convoluted tubules of the cortex 2 hr after injection.
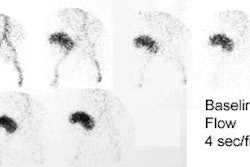
a. 40 to 65% of the radiopharmaceutical is handled by glomerular filtration permitting dynamic imaging.
b. Acquisition of dynamic and static images may be performed immediately and within 20 to 30 min after the injection of the
radiopharmaceutical.
3. Delayed imaging is suggested 2 to 4 hr after the injection of the radiopharmaceutical. With poor renal function or clearance, even further delayed imaging will increase lesion definition.
4. Delayed imaging (up to 24 hr after the injection) may be necessary for quantitation of split renal function when there is a severely obstructed collecting system.
5. The renal cortical exposure to the kidneys is equivalent for both radiopharmaceuticals if there is adherence to the suggested doses. DMSA provides a lower gonadal and bladder exposure.
6. Hepatic and biliary activity may be a problem in patients with poor renal function in renal cortical scintigraphy.
7. The minimal administered activity for Tc-99m DMSA is about 10 MBq (0.3 mCi). The maximum administered activity for Tc-99m DMSA is about 110 MBq (3.0 mCi).
8. The minimal administered activity for Tc-99m GH is about 20 MBq (0.5 mCi). The maximum administered activity for Tc-99m GH is about 300 MBq (8.0 mCi).
E. Image Acquisition:
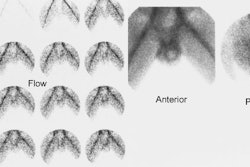
1. Posterior and posterior oblique parallel hole high-resolution or ultra high-resolution images of both kidneys are obtained for 300,000 to 500,000 counts on a 128 x 128 or 256 x 256 matrix format. Analog images are also acceptable.
2. Posterior and posterior oblique pinhole collimation (2 to 3 mm aperture) imaging with high-resolution collimator magnification of each kidney is obtained for approximately 150,000 counts (15 – 20 min per image) using a 128 x 128 or 256 x 256 matrix. This allows detection of smaller cortical defects.
3. Horseshoe and pelvic kidneys are better defined when imaged anteriorly to detect the connecting bridge of renal tissue anterior to the spine.
a. In patients who have a history of a high congenital spinal defect (e.g. meningomyelocele), horseshoe and pseudohorseshoe kidneys lie in the deep kyphotic fossa and the examination should then be performed with the patient in the prone or lateral decubitus position.
b. In these patients, horseshoe and pseudohorseshoe kidneys lie in the deep kyphotic fossa and are better defined on posterior imaging than anterior imaging.
4. SPECT requires sampling over 360 degrees usually on a 128 x 128 matrix. A multi-headed camera may reduce the imaging time, providing time and counts remain the same.
F. Interventions
1. An indwelling venous catheter may be necessary in younger children (less than four years of age) or in older children unable to cooperate in remaining motionless for a prolonged period of time. The venous access allows injection of the radiopharmaceutical as well as the injection of intravenous sedation or diuretic, if needed, without the necessity for additional percutaneous injections.
2. The use of cortical agents allows either vesicoureteral reflux or retained activity in the collecting systems to interfere with the interpretation of the percent differential renal function.
a. Retained activity in the collecting system can be caused by a back pressure effect of a very distended neurogenic bladder, and may be prevented by catheterization and continuous drainage.
b. In the case of a capacious collecting system or obstructed system, a diuretic (furosemide) can be administered prior to the delayed imaging, or the patient can return for imaging 24 hr after the injection of the radiopharmaceutical.
G. Processing
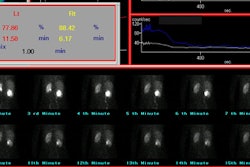
For determination of percent differential function, regions of interest of each kidney and background areas are outlined on the computerized posterior image.
H. Interpretation Criteria
1. DMSA localization is usually not seen in the medulla or the collectingsystem.
2. The split function normally varies from 50–50% to 44–56% (one kidney
compared to the other).
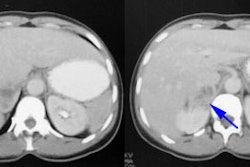
3. Acute pyelonephritis may appear as single or multiple defects.
a. The cortical defect may have reduced or absent localization of tracer with indistinct margins that don’t deform the renal contour.
b. A localized increase in volume of a single affected area or a diffusely enlarged kidney with multiple defects may occur.
4. A mature cortical scar may have relatively sharp edges with contraction and reduced volume of the affected cortex.
a. Scarring can manifest as cortical thinning, flattening or an ovoid or wedge-shaped defect.
b. The defect may become more obvious with growth of normal surrounding cortex.
c. The scan defect related to acute pyelonephritis may resolve in a variable period of time depending on the severity of infection. A follow-up of 3 to 6 mo may be recommended after therapy.
5. Acute and chronic pyelonephritis or scarring cannot always be distinguished on the cortical scan.
I. Reporting
1. The procedure, the date of the study, amount and route of administration of the radiopharmaceutical (include diuretic if administered), and previous study for comparison study are included.
2. The history includes the symptoms and/or diagnosis.
3. The technique includes time of imaging as well as pinhole collimation or SPECT.
4. The findings should include the position, size and overall morphology of functioning renal tissue; split renal function; the size, number and location of areas of cortical loss; and the distinguishing of acute versus chronic pyelonephritis, if possible.
J. Quality Control
Air introduced into reaction vial can degrade the DMSA complex, resulting in decreased renal uptake and increased hepatic and background activity.
K. Sources of Error
1. The flattening of the superolateral aspect of the left kidney may be attributed to the splenic impression.
2. The linear area of absent tracer uptake (interrenicular septum) extending from the renal hilum into the parenchyma is seen on SPECT and may be confused with scar.
V. Issues Requiring Further Clarification
Additional studies with DMSA are needed to determine the clinical value of planar scintigraphy, pinhole scintigraphy and SPECT in the evaluation of patients suspected of having pyelonephritis.
VI. Concise Bibliography
A. American Academy of Pediatrics, Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 1992;89:1110-1115.
B. Benador D, Benador N, Slosman DO, et al. Cortical scintigraphy in the evaluation of renal parenchymal changes in children with pyelonephritis. J Pediatr 1994;124:17-20.
C. Jakobsson B, Nolstedt L, Svensson L, et al. 99m Technetium-dimercaptosuccinic acid scan in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol 1992;6:328-334.
D. Joseph DB, Young DW, Jordon SP. Renal cortical scintigraphy and single photon emission computed tomography (SPECT) in the assessment of renal defects in children. J Urol 1990;144:595-597.
E. Kass EJ, Fink-Bennett D, Cacciarelli AA, et al. The sensitivity of renal scintigraphy and sonography in detecting nonobstructive acute pyelonephritis. J Urol 1992;148:606-608.
F. Longergan GJ, Pennington DJ, Morrison JC, et al. Childhood pyelonephritis: comparison of gadolinium-enhanced MR imaging and renal cortical scintigraphy for diagnosis. Radiology 1998;207:377-384.
G. Montgomery P, Kuhn JP, Afshani E. CT evaluation of severe renal inflammatory disease in children. Pediatr Radiol 1987;17:216-222.
H. Rushton HG, Majd M, Jantausch B, et al. Renal scarring follow reflux and nonreflux pyelonephritis in children: evaluation with 99m Technetium-dimercaptosuccinic acid scintigraphy. J Urol 1992;147:1327-1332.
I. Tarkington MA, Fildes RD, Levin K, et al. High resolution single photon emission computed tomography (SPECT) 99m Technetium-dimercaptosuccinic acid renal imaging: a state of the art technique. J Urol 1990;144:558-560.
J. Taylor A, Lallone RL, Hagan PL. Optimal handling of dimercaptosuccinic acid for quantitative renal scanning. J Nucl Med 1980;21:1190-1193.
K. Van Luyk WHU, Ensing GJ, Piers DA. Low renal uptake of Tc-99m DMSA in patients with proximal tubular dysfunction. Eur J Nucl Med 1983;8:404-405.
VII. Disclaimer
The Society of Nuclear Medicine has written and approved guidelines to promote the cost-effective use of high quality nuclear medicine procedures. These generic recommendations cannot be applied to all patients in all practice settings. The guidelines should not be deemed inclusive of all proper procedures or exclusive of other procedures reasonably directed to obtaining the same results. The spectrum of patients seen in a specialized practice setting may be quite different than the spectrum of patients seen in a more general practice setting. The appropriateness of a procedure will depend in part on the prevalence of disease in the patient population. In addition, the resources available to care for patients may vary greatly from one medical facility to another. For these reasons, guidelines cannot be rigidly applied.
Advances in medicine occur at a rapid rate. The date of a guideline should always be considered in determining its current applicability.