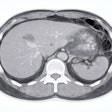
RevealDx has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its RevealAI-Lung software for characterizing nodules.
The software, validated on over 1,500 patients across a range of cohorts, characterizes nodules by producing a Malignancy Similarity Index (mSI) score to help radiologists make informed follow-up recommendations for cancer diagnosis.
The software can be directly integrated into PACS systems, according to RevealDx.
With this clearance, RevealAI-Lung is now reimbursable using codes 0721T and 0722T, with a current Medicare payment rate in OPPS of $650, RevealDx noted.
RevealAI-Lung received EU Medical Device Regulation (MDR) Certification in November 2025.
The software can be purchased directly from the firm or through its U.S. distributor Sirona, the company said.