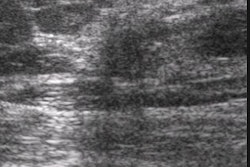
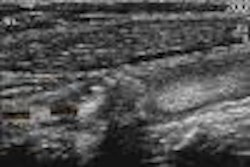
The Food and Drug Administration has given 510(K) clearance to the Ultrasonix 500 ultrasound imaging scanner, developed by Ultrasonix of Vancouver, British Columbia. The system received its Canadian approval in May, and is moving toward regulatory clearance in Asia.
The company said it expects to start shipping products in the third quarter in North America. The Ultrasonix 500 is slated for competitive pricing as a platform suited for shared services clinics requiring one system to be used in different applications.
Ultrasonix 500 is the first of a series of highly mobile, small-footprint products based on a new architecture, according to Ultrasonix. Ultrasonix 500 was introduced as a work in progress at the 2002 American Institute of Ultrasound in Medicine meeting in Nashville, TN.
By AuntMinnie.com staff writers
June 27, 2002
Copyright © 2002 AuntMinnie.com